CAR-T Approval Updates: Moving up the Rung in Multiple Myeloma and Breaking Ground in Chronic Lymphocytic Leukemia
Authors: Michael Hardler, PharmD, BCOP; Tyler Sandahl, PharmD, BCOP; and Rachel Bailen, PharmD, BCOP, BCPS, Hematology/Oncology Clinical Pharmacists at Mayo Clinic in Rochester, Minnesota
Introduction: Recent Advances in CAR-T Therapies
Chimeric Antigen Receptor T-cell (CAR-T) therapy indications continue to expand with recent updates to CAR-T products for treatment of Multiple Myeloma (MM), Chronic Lymphocytic Leukemia / Small Lymphocytic Lymphoma (CLL/SLL), Follicular Lymphoma (FL), and Mantle Cell Lymphoma (MCL) (Table 1). The U.S. Food and Drug Administration (FDA) has approved the use of ciltacabtagene autoleucel (Carvykti®; cilta-cel) and idecabtagene vicleucel (Abecma®; ide-cel) for earlier treatment of adults with MM. Both cilta-cel and ide-cel are B-Cell Maturation Antigen (BCMA)-targeting CAR-T products with prior approvals in the relapsed/refractory (RR) multiple myeloma (RRMM) space. CLL/SLL patients have their first CAR-T therapy as an accelerated approval by the FDA for lisocabtagene maraleucel (Breyanzi®; liso-cel), a CD-19 targeted CAR-T. Additionally, liso-cel had approvals in FL and MCL confirmed this past May, but we’ll save full discussion of FL and MCL indication approvals for another time.
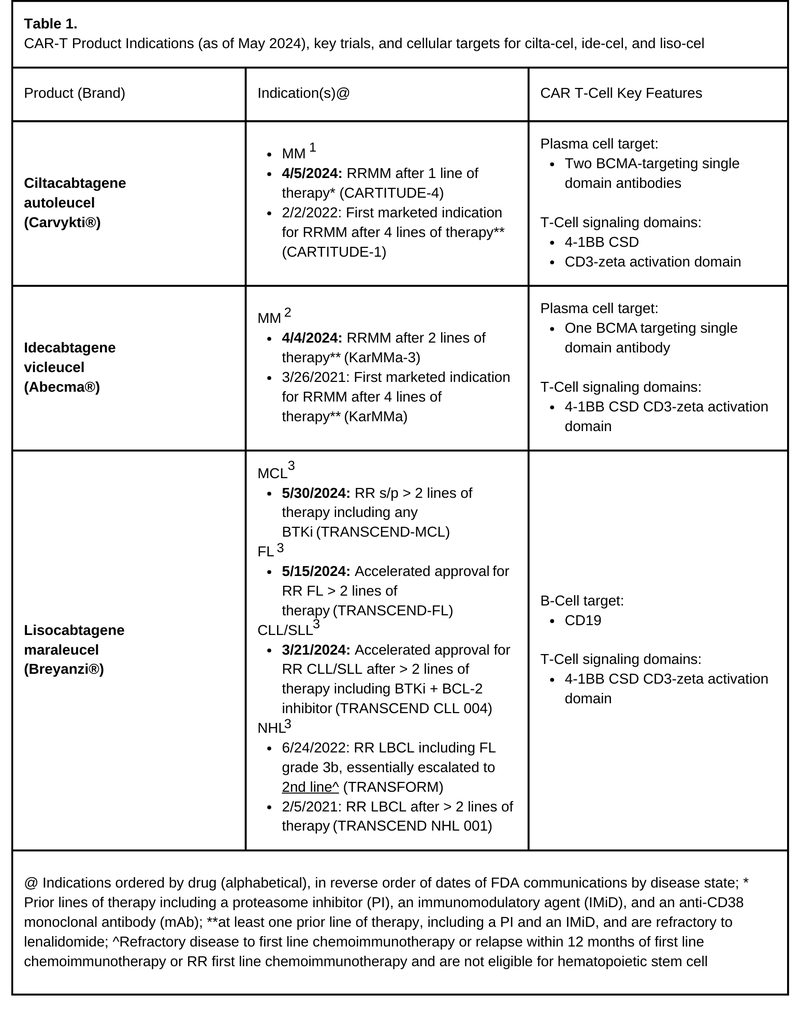
Cilta-cel and Ide-cel Updates: Expanding the Reach of CAR-T for Multiple Myeloma Patients
The first week of April 2024 brought two new FDA approvals advancing the place in therapy for both cilta-cel and ide-cel, allowing for earlier treatment in adults with RRMM. Both products were previously approved for RRMM after four lines of therapy including a proteasome inhibitor (PI), an immunomodulatory drug (IMiD), and an anti-CD38 monoclonal antibody (mAb). Based on the results of the KarMMA-3 trial, ide-cel is now approved for RRMM after two lines of therapy including a PI, IMID, and anti-CD38 mAb2,4. Cilta-cel gained approval for use in RRMM after one line of therapy including a PI and IMiD who are refractory to lenalidomide based on findings in CARTITUDE-41,5.
In CARTITUDE-4, investigators compared cilta-cel (n=208) versus investigators choice of two standard of care regimens (n=211), daratumumab, pomalidomide, and dexamethasone (DPd; n=183), or pomalidomide, bortezomib, and dexamethasone (PVd; n=26) for lenalidomide-refractory patients who had received 1 to 3 previous lines of treatment.4 The proportion of patients with prior autologous-hematopoietic stem cell transplant (HSCT) is not reported independently for each arm but was 85% in the overall study population.1 With a median follow-up of 15.9 months, the median progression-free survival (PFS) wasn’t yet reached in the cilta-cel arm compared to 11.8 months in the DPd/ PVd standard care group. The cilta-cel cohort showed a superior overall response rate (84.6% vs 67.3%) versus PVd/DPd and the depth of response favored cilta-cel cohort with complete response rates or better of 73.1 % vs 21.8% and absence of minimal residual disease (60.6% vs 15.6%). The safety and toxicity profile for cilta-cel was similar to those previously reported.4
The KarMMa-3 trial included triple-class exposed RRMM patients previously treated with 2 to 4 lines of therapy (including a PI, an IMiD, and an anti-CD38 mAb), comparing ide-cel (n=254) against investigators choice of five standard of care regimens (n=132). Standard of care options included DPd (n=41), elotuzumab, pomalidomide, and dexamethasone (EPd; n=30), carfilzomib and dexamethasone (Kd; n=28), ixazomib, lenalidomide, and dexamethasone (IRd; n=20), or daratumumab, bortezomib, and dexamethasone (DVd; n=7). At a median follow-up of 18.6 months the median PFS was 13.3 months in the ide-cel group versus 4.4 months in the standard-care group (HR=0.49) improving PFS by three-fold. Response rates were 71% vs 42% in ide-cel vs standard-care respectively, continuing to show the achievability of clinically significant responses with CAR-T agents even in more heavily pre-treated populations. The safety and toxicity profile for ide-cel was similar to those previously reported.5
KarMMa-3 patients were heavily pre-treated (median lines of therapy was 3; range 2-4) with triple-class refractory patients accounting for 65-67% of the study population and penta-class refractory patients in 4-6% of patients. Additionally, 66% (n=167) of patients in the ide-cel arm and 66% (n=87) in the standard of care arm had one prior auto-HSCT and nearly 20% in each arm had more than one prior auto-HSCT, yet still achieved deep and durable responses.5 While response rates appear more impressive with cilta-cel, attempting cross trial comparison is inherently difficult and can be misleading.
For transplant-eligible patients, the most likely place in therapy for CAR-T now is post-transplant with relapsed/refractory disease. Auto-HSCT is generally preferred first-line for eligible patients, so whether it is given upfront or delayed (2nd-line or later), it is conceivable that almost all transplant-eligible patients receiving cilta-cel or ide-cel will have received prior transplant – at least, until CARTITUDE-6, which may suggest otherwise.
For transplant-ineligible patients, cilta-cel and ide-cel are both highly effective options for RRMM. Cilta-cel, now approved for 2nd line therapy, and ide-cel, now approved for 3rd line therapy, can fulfil a need for a potent therapy in patients unfit for transplant and patients interested in having more time without treatment in earlier lines of therapy. CAR-T toxicities can be life-threatening; however, it is still considered to be more tolerable than transplant in frail patients.
Overall, NCCN supports both new product indications as category 1 recommendations in agreement with the FDA approval criteria described for ide-cel and cilta-cel.6 Mayo Stratification for Myeloma And Risk-adapted Therapy (mSMART) guidelines offer that CAR-T can be considered as an option for triple class refractory patients at first or early relapse after quadruplet therapy induction and auto-HSCT. The mSMART algorithm showcases a bit more of a conservative introduction of CAR-T for now, which highlights that not all practices will utilize CAR-T second line for all patients without longer term follow-up data.7 It’s likely that some hesitance to elevate CAR-T products in lines of therapy comes from concern for potentially severe and long-term toxicities of CAR-T. Data regarding prevalence of such risks as secondary malignancies and myelodysplastic syndromes have yet to mature enough to assess prevalence, but as we have seen with the recent FDA-mandated update to CAR-T labels regarding risks of as T-Cell malignancies, our understanding of long-term toxicities with CAR-T is likely still in its infancy and will continue to evolve.8
CAR-T Breaks Ground in the CLL Realm with liso-cel
Over the last decade, therapies for CLL/SLL have significantly improved. Frontline therapy currently consists of a Bruton’s tyrosine kinase inhibitor (BTKi) or venetoclax plus obinutuzumab.9-11 While most patients will achieve partial or complete remission with these frontline therapies, patients will ultimately relapse necessitating subsequent lines of therapy. Treatment options for patients with RR CLL/SLL after treatment with both BTKi and/or venetoclax currently include pirtobrutinib (a non-covalent BTKi) or Phosphatidylinositol 3-kinase inhibitors (idelalisib and duvelisib - generally limited by poor tolerance). Patients with RR disease may elect to undergo allogeneic-HSCT, which may offer cure, but risks often outweigh the benefit of potential cure in older or frail patients. Therefore, CAR-T therapies may have a role to play in CLL patients, especially those who are transplant-ineligible or with RR disease post-transplant.12,13
A few single-center studies have evaluated CD19-directed CAR-T products in patients with RR CLL and generally showed high rates of severe toxicities, lower response rates, and lower median PFS compared to other malignancies.14-16 However, patients that achieve a CR seem to achieve long-term remission, suggesting that depth of response is integral for long-term clinical benefit.14 Investigators of TRANSCEND CLL 004 were able to achieve seemingly more rapid and deeper responses than prior comparators.17
The FDA granted accelerated approval for liso-cel in CLL based on the TRANSCEND CLL 004, phase 1/2 trial, with the ongoing phase 2 trial for patients at a dose level of 100 x 106 CAR-T cells.18 Investigators included patients with standard-risk features with > 3 prior therapies or high-risk features treated with > 2 prior therapies including a BTKi. Most patients (83%) had high-risk features (including TP53 and del(17p)). While this trial analyzed several different patient subsets, the primary endpoint was complete response or remission in patients with previous BTKi progression and venetoclax failure (n=49). The primary endpoint was achieved in 9 of those patients (18%). Overall response rate in this subset, a secondary endpoint, was found in 21 patients (43%), which was not statistically significant. Undetectable MRD rate was 63% (in blood) and 59% (in marrow) with a median time to first complete response or remission of 3 months. Median duration of response overall was 35.3 months, but in the patients who achieved complete response or remission, duration of response was not reached. As in earlier studies, this shows that while the number of CLL patients who achieve CR with CAR-T may be numerically lower than other indications, those who do achieve CR seem to have long remissions. Unfortunately, current data does not allow the ability to predict who will achieve CR based on patient characteristics. Importantly, the data available from Phase 1/2 trial has prompted NCCN recommendation updates, which now include liso-cel as a category 2A recommendation for RR CLL/SLL patients after prior BTKi- and venetoclax-based regimens.19
These results led to the FDA accelerated approval for patients with refractory CLL/SLL who had received at least two prior lines of therapy including a BTKi and a B-cell lymphoma-2 inhibitor (BCL-2i). Notably, continued approval is contingent on continued clinical benefit being shown in confirmatory trials. For now, liso-cell provides an additional line of therapy for RR CLL patients.
Summary
On top of the previously discussed approvals, liso-cel has been granted with FDA approvals for both FL and MCL as well. Data from TRANSCEND-FL (NCT04245839) prompted FDA accelerated approval of liso-cel for adult patients with RR FL who have received two or more prior lines of systemic therapy, marking it the third CAR-T in this space after axicabtagene ciloleucel (Yescarta®) and tisagenlecleucel (Kymriah®). Furthermore, data from TRANSCEND-MCL led to FDA approval for use of liso-cel in adult patients with RR MCL who have received at least two prior lines of systemic therapy, including a BTKi – infiltrating a space where only one other CAR-T therapy, brexucabtagene autoleucel (Tecartus®) was previously available.20
Overall, these updates offer exciting steps forward for patients with hematologic malignancies, but there’s certainly more work to be done. Future questions surrounding CAR-T in the MM space is whether CAR-T will overtake auto-HSCT as preferred consolidation in 1st line therapy in NDMM or if it will remain a 2nd line option. Although it seems likely to be a few years away from study completion, CARTITUDE-6 has begun recruiting and will look at cilta-cel versus auto-HSCT in NDMM.4 KarMMa-7 (NCT04855136) will explore use of ide-cel in combination with either iberdomide and dexamethasone or BMS-986405 (oral inhibitor of integral membrane protein gamma-secretase). For CLL/SLL, the anti-cancer community eagerly await further results from the TRANSCEND CLL 004 phase 2 trial, hopefully showing benefit that secures the liso-cel approval for this patient population.
References
1.Janssen Biotech. Carvykti (ciltacabtagene autoleucel) [package insert]. U.S. Food and Drug Administration website. https://www.fda.gov/media/156560/download?attachment. Revised [April 2024]. Accessed [6/10/2024].
2.Celgene Corporation, a Bristol-Myers Squibb Company. Abecma (idecabtagene vicleucel) [package insert]. U.S. Food and Drug Administration website. https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel. Revised [April 2024]. Accessed [6/10/2024].
3.Juno Therapeutics Inc., a Bristol-Myers Squibb Company, Celgene Corporation, a Bristol-Myers Squibb Company and Bristol-Myers Squibb Company. Breyanzi (lisocabtagene maraleucel) [package insert]. U.S. Food and Drug Administration website. https://www.fda.gov/media/145711/download?attachment. Revised [May 2024]. Accessed [6/10/2024].
4.San-Miguel J, Dhakal B, Yong K, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. N Engl J Med. Jul 27 2023;389(4):335-347. doi:10.1056/NEJMoa2303379
5.Rodriguez-Otero P, Ailawadhi S, Arnulf B, et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N Engl J Med. Mar 16 2023;388(11):1002-1014. doi:10.1056/NEJMoa2213614
6.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Multiple Myeloma V.4.2024 - April 26, 2024. © National Comprehensive Cancer Network, Inc. 202X. All rights reserved. Accessed [7/2/2024]. To view the most recent and complete version of the guideline, go online to NCCN.org.
7.Dingli D, Ailawadhi S, Bergsagel PL, et al. Therapy for Relapsed Multiple Myeloma: Guidelines From the Mayo Stratification for Myeloma and Risk-Adapted Therapy. Version 8. Last reviewed May 2024. Accessed [7/2/2024]. Available online at https://www.msmart.org/mm-treatment-guidelines. . Mayo Clin Proc. Apr 2017;92(4):578-598. doi:10.1016/j.mayocp.2017.01.003
8.Center for Biologics Evaluation and Research. 2024 safety and Availability Communications. FDA Requires Boxed Warning for T cell Malignancies Following Treatment with BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies. U.S. Food and Drug Administration. April 18, 2024. Accessed July 2nd, 2024. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-boxed-warning-t-cell-malignancies-following-treatment-bcma-directed-or-cd19-directed.
9.Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. Apr 18 2020;395(10232):1278-1291. doi:10.1016/S0140-6736(20)30262-2
10.Tam CS, Brown JR, Kahl BS, et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial. Lancet Oncol. Aug 2022;23(8):1031-1043. doi:10.1016/S1470-2045(22)00293-5
11.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N Engl J Med. Jun 6 2019;380(23):2225-2236. doi:10.1056/NEJMoa1815281
12.Hampel PJ, Parikh SA. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer Journal. 2022/11/29 2022;12(11):161. doi:10.1038/s41408-022-00756-9
13.Burger JA. Treatment of Chronic Lymphocytic Leukemia. N Engl J Med. Jul 30 2020;383(5):460-473. doi:10.1056/NEJMra1908213
14.Turtle CJ, Hay KA, Hanafi LA, et al. Durable Molecular Remissions in Chronic Lymphocytic Leukemia Treated With CD19-Specific Chimeric Antigen Receptor-Modified T Cells After Failure of Ibrutinib. J Clin Oncol. Sep 10 2017;35(26):3010-3020. doi:10.1200/JCO.2017.72.8519
15.Geyer MB, Riviere I, Senechal B, et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. Apr 2 2019;5(9)doi:10.1172/jci.insight.122627
16.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. Sep 2 2015;7(303):303ra139. doi:10.1126/scitranslmed.aac5415
17.Siddiqi T, Maloney DG, Kenderian SS, et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): a multicentre, open-label, single-arm, phase 1–2 study. The Lancet. 2023;402(10402):641-654. doi:10.1016/S0140-6736(23)01052-8
18.Siddiqi T, Soumerai JD, Dorritie KA, et al. Phase 1 TRANSCEND CLL 004 study of lisocabtagene maraleucel in patients with relapsed/refractory CLL or SLL. Blood. Mar 24 2022;139(12):1794-1806. doi:10.1182/blood.2021011895
19.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Lymphocytic Leukemia/ Small Lymphocytic Lymphoma V.3.2024 - March 26, 2024. © National Comprehensive Cancer Network, Inc. 202X. All rights reserved. Accessed [7/2/2024]. To view the most recent and complete version of the guideline, go online to NCCN.org.
20.Wang M, Siddiqi T, Gordon LI, et al. Lisocabtagene Maraleucel in Relapsed/Refractory Mantle Cell Lymphoma: Primary Analysis of the Mantle Cell Lymphoma Cohort From TRANSCEND NHL 001, a Phase I Multicenter Seamless Design Study. J Clin Oncol. Apr 1 2024;42(10):1146-1157. doi:10.1200/JCO.23.02214



