How Low Can You Go: Fam-trastuzumab deruxtecan-nxki for HER2 Ultralow Metastatic Breast Cancer
Authors
- Allison Snoke, PharmD, BCOP, Clinical Pharmacy Specialist Hematology/Oncology at Intermountain Health, Murray, UT
- Danielle Gundrum, PharmD, BCOP, Clinical Pharmacy Specialist Hematology/Oncology at Intermountain Health, Murray, UT; and Associate Professor of Pharmacy Practice at Roseman University of Health Sciences - College of Pharmacy, South Jordan, UT
Background
Breast cancer is the most common occurring cancer in females in the United States and is the second leading cause of cancer death among this population.1 In 2024, it is estimated that 313,510 new cases of invasive breast cancers will be diagnosed and along with 42,780 breast cancer-related deaths.
Approximately 20% of breast cancers are human epidermal growth factor receptor 2 (HER2) positive.2, 3 HER2 is a transmembrane glycoprotein epidermal growth factor receptor (EGFR) with tyrosine kinase activity that is expressed on the surface of numerous tumor-types, including breast cancer.2 In breast cancer patients, HER2 positivity is associated with worse prognosis due to increased risk of recurrence.2 Patients who are determined to be HER2 positive may benefit from monoclonal antibodies and/or tyrosine kinase inhibitors designed to target HER2 signaling pathways.4, 5
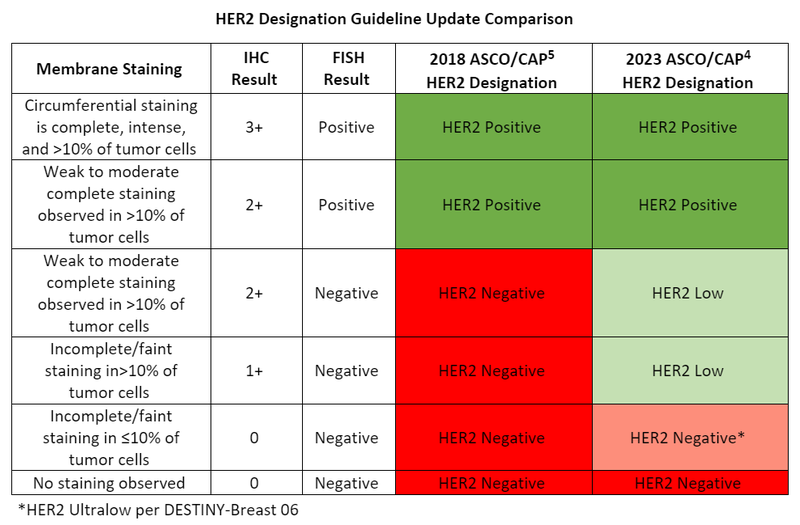
Per the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) HER2 testing in breast cancer guidelines, the expression of HER2 on breast cancer cells is evaluated by an immunohistochemistry (IHC) test.4, 5 A test result of 3+ (circumferential membrane staining that is complete, intense and in >10% of tumor cells) determines the cancer is HER2 positive. For HER2 negativity, an IHC result of 0 (no staining observed or incomplete/faint staining in ≤10% of tumor cells) or 1+ (incomplete/faint staining in>10% of tumor cells) is required.4, 5 An IHC result of 2+ (weak to moderate complete staining observed in >10% of tumor cells) is regarded as equivocal meaning an additional fluorescence in situ hybridization (FISH) test must be done to further determine HER2 positivity or negativity.4, 5
Due to the publication of the DESTINY-Breast 04 trial in 2022, the ASCO/CAP HER2 testing in breast cancer guideline required updating in 2023 to address the introduction of the HER2 low category.4, 6 HER2 low is defined by an IHC of 1+ or an IHC of 2+ with negative FISH results meaning some patients who were originally classified as HER2 negative are now “low positive” and have the option of getting treated with a HER2-directed antibody thereby broadening treatment options.4, 6
The evolution continues this year with the introduction of HER2 ultralow breast cancer which captures even lower levels of HER2 due to the DESTINY-Breast 06 study.7 HER2 ultralow is defined by IHC 0 with membrane staining in ≤10% of tumor cells.7 The drug at the treatment forefront of HER2 low, and most recently ultralow, is fam-trastuzumab deruxtecan-nxki.6-8
DESTINY-Breast 06
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody-drug conjugate that is approved for the treatment of HER2 positive and more recently HER2 low unresectable or metastatic breast cancer.9 Trastuzumab is covalently linked to the topoisomerase 1 inhibitor deruxtecan.9 Once trastuzumab binds to HER2 receptors on the cancer cell, the complex is internalized where the deruxtecan portion is cleaved causing DNA damage and ultimately cell death.9 In 2022, fam-trastuzumab deruxtecan-nxki was granted approval for the treatment of HER2 low metastatic breast cancer based off the results of the DESTINY-Breast 04 trial.6, 9 In comparison to physician's choice of chemotherapy, fam-trastuzumab deruxtecan-nxki resulted in increased progression free and overall survival (OS).6
The FDA has now granted priority review in the United States to fam-trastuzumab deruxtecan-nxki for patients with HER2 low or ultralow metastatic breast cancer who have received at least one line of endocrine therapy based off primary results from the DESTINY-Breast 06 trial.7, 10 This study was a randomized, open label, phase 3 trial that evaluated the safety and efficacy of fam-trastuzumab deruxtecan-nxki versus investigator’s choice of chemotherapy in patients with hormone positive, HER2 low or ultralow, advanced, or metastatic breast cancer. Of note, patients received no prior chemotherapy in this setting but received at least two prior lines of endocrine therapy or one line but had disease recurrence within 24 months after initiation. HER2 low was defined as IHC 1+ or IHC 2+ and negative FISH results. HER2 ultralow was defined as IHC 0 with membrane staining further defined as IHC>0 and <1+. Patients were randomized in a 1:1 ratio to receive fam-trastuzumab deruxtecan-nxki 5.4 mg/kg once every 3 weeks or investigator’s choice of single-agent chemotherapy which consisted of paclitaxel, nab-paclitaxel, or capecitabine. The primary endpoint was progression free survival (PFS) in the HER2 low population. Secondary end points included PFS in the intent to treat (ITT) population, OS in the HER2 low and ITT population, as well as safety. Analysis in patients with HER2 ultralow was exploratory. As of March 2024, 866 patients were randomized (n=713 for HER2 low and n=153 for HER2 ultralow). Baseline characteristics were well balanced between both groups.
At the data-cutoff date, PFS according to blinded independent central review was improved in the HER2 low disease population, with the median PFS duration of 13.2 months in the fam-trastuzumab deruxtecan-nxki group versus 8.1 months in the chemotherapy group (hazard ratio (HR) for disease progression or death, 0.62; 95% CI, 0.51 to 0.74; P<0.001). Results were consistent in the exploratory HER2 ultralow population with a median PFS of 13.2 months in the fam-trastuzumab deruxtecan-nxki group and 8.3 months in the chemotherapy group (HR for disease progression or death, 0.78; 95% CI, 0.50 to 1.21). In the ITT population, PFS was significantly longer in the HER2 low population; the fam-trastuzumab deruxtecan-nxki group proved a median PFS of 13.2 months versus 8.1 months in the chemotherapy arm (HR for disease progression or death, 0.63; 05% CI, 0.53 to 0.75; P<0.001).
OS at the data-cutoff date showed 37.9% maturity in the fam-trastuzumab deruxtecan-nxki group versus 41.2% maturity in the chemotherapy group. The difference in OS between treatment groups was not statistically significant in the HER2 low population (HR for death, 0.83, 95% CI, 0.66 to 1.05) with an estimated 12-month OS of 87.6% in the fam-trastuzumab deruxtecan-nxki group versus 81.7% in the chemotherapy group. Consistent results were observed in the ITT and HER2 ultralow populations.
The safety analysis included a total of 851 patients: 434 in fam-trastuzumab deruxtecan-nxki group and 417 in the chemotherapy group. The incidence of adverse events that occurred were similar between treatment groups with 98.8% observed in the fam-trastuzumab deruxtecan-nxki group and 95.2% in the chemotherapy group. Grade 3 or higher events were reported in 52.8% of the fam-trastuzumab deruxtecan-nxki group compared to 44.4% in the chemotherapy group. Fatal adverse events occurred in 1.2% of the fam-trastuzumab deruxtecan-nxki group (5 patients). Zero fatal adverse events occurred in the chemotherapy group. Interstitial lung disease or pneumonitis occurred in 11.3% of the fam-trastuzumab deruxtecan-nxki group versus 0.2% in the chemotherapy group.
DESTINY-Breast 06 investigators concluded HER2 low or HER2 ultralow metastatic breast cancer patients who received fam-trastuzumab deruxtecan-nxki after one or more lines of endocrine-based therapy experienced longer PFS when compared to chemotherapy, and no new safety concerns were identified.
Discussion/Conclusion
The ability of fam-trastuzumab deruxtecan-nxki to treat breast cancer patients with HER2 IHC 0 with positive membrane staining up to IHC 3+ has changed the treatment paradigm for metastatic disease. Approximately 60-65% of patients who are hormone positive, HER2 negative express low levels of HER2 and another 25% are estimated to be HER2 ultralow.3 The current standard of care for these patients consists of an endocrine based regimen usually coupled with a CDK4/6 inhibitor as first line treatment.7 After CDK 4/6 inhibitor treatment, there is no consensus on sequencing of therapy upon disease progression.7 Fam-trastuzumab deruxtecan-nxki is thought to be more effective than older HER2 targeting agents in treating HER2 low and ultralow levels because of the bystander effect.7 This mechanism results from the deruxtecan portion of the antibody drug conjugate permeating the cell membrane and killing tumor cells in close proximity regardless of HER2 expression.7
The DESTINY-Breast 04 trial established the efficacy of fam-trastuzumab deruxtecan-nxki in HER2 low disease after receiving chemotherapy.6 In the DESTINY-Breast 06 trial, fam-trastuzumab deruxtecan-nxki resulted in longer PFS in comparison to chemotherapy in earlier lines of treatment for metastatic cancer following endocrine based therapy.7 Therefore, in metastatic disease, fam-trastuzumab deruxtecan-nxki may be utilized as an additional treatment option between endocrine therapy and standard chemotherapy for patients who have received one or more lines of endocrine treatment. Although the study was not powered to demonstrate statistical significance in the HER2 ultralow population, the data in this population was consistent with both the ITT and HER2 low populations. No new safety events were identified, but the risk of interstitial lung disease remains prominent.
Currently, there is no need to discern between HER2 low and ultralow cancers because of the consistent benefit observed in both groups despite the limited sample size in the ultralow population.7 The main shift in clinical practice will be in regard to separating the HER2 IHC 0 with positive membrane staining (HER2 ultralow) and IHC 0 without membrane staining (HER2 negative). Currently, it is not standard practice to distinguish between the two per the 2023 ASCO-CAP guideline update.4 Clinical validation of reference ranges and diagnostics for HER2 ultralow disease will need to be evaluated and established in the future.3
Per Ian Krop, MD, PhD, the Director of the Yale Cancer Center Clinical Trials Office and Chief Clinical Research Officer, “Current IHC testing is relatively poor at distinguishing HER2 low and ultralow cancers from HER2 0 cancers… There are probably multiple reasons for this, but an important one is that the original test was designed to distinguish high levels of HER2 [IHC 3+] from all the lower levels. It was not designed to distinguish the very low levels to the even lower or 0 cancers.”11
Overall, the DESTINY-Breast 06 trial continues to support the preexisting HER2 low PFS benefit data. Down the line, an expanded approval of fam-trastuzumab deruxtecan-nxki for HER2 ultralow disease would offer an additional line of therapy in treatment algorithms for patients who previously would not have been previously eligible.
References
1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. Jan-Feb 2024;74(1):12-49. doi:10.3322/caac.21820
2.Corti C, Giugliano F, Nicolò E, Tarantino P, Criscitiello C, Curigliano G. HER2-Low Breast Cancer: a New Subtype? Curr Treat Options Oncol. May 2023;24(5):468-478. doi:10.1007/s11864-023-01068-1
3.Enhertu Demonstrated a Median Progression-Free Survival of 13.2 Months in HR-Positive, HER2-Low and HER2-Ultralow Metastatic Breast Cancer Following One or More Lines of Endocrine Therapy. June 2, 2024. Accessed November 23, 2024. www.astrazeneca.com/media-centre/press-releases/2024/Enhertu-demonstrated-median-progression-free-survival-thirteen-months.html
4.Wolff AC, Somerfield MR, Dowsett M, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology–College of American Pathologists Guideline Update. Archives of Pathology & Laboratory Medicine. 2023;147(9):993-1000. doi:10.5858/arpa.2023-0950-SA
5.Wolff AC, Hammond MEH, Allison KH, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. Jul 10 2018;36(20):2105-2122. doi:10.1200/jco.2018.77.8738
6.Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New England Journal of Medicine. 2022;387(1):9-20. doi:doi:10.1056/NEJMoa2203690
7.Bardia A, Hu X, Dent R, et al. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. New England Journal of Medicine. 2024. doi:doi:10.1056/NEJMoa2407086
8.Giuseppe Curigliano et al. Trastuzumab deruxtecan (T-DXd) vs physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). JCO 42, LBA1000-LBA1000(2024). doi:10.1200/JCO.2024.42.17_suppl.LBA1000
9.Fam-Trastuzumab Deruxtecan. Lexi-Drugs. UpToDate Lexidrug. UpToDate Inc. Accessed November 25. https://online.lexi.com
10.Enhertu granted priority review in the US for patients with HER2-low or HER2-ultralow metastatic breast cancer who have received at least one line of endocrine therapy. October 1, 2024. Astrazeneca.com. Accessed November 23, 2024. https://www.astrazeneca.com/media-centre/press-releases/2024/enhertu-granted-priority-review-us-for-patients-her2-low-or-her2-ultralow-metastatic-breast-cancer-who-have-received-at-least-1-line-endocrine-therapy.html
11.Ryan C. Trastuzumab Deruxtecan Prolongs PFS in Pretreated HR+, HER2-Low and -Ultralow Metastatic Breast Cancer. June 2, 2024. Accessed November 5, 2024. https://www.onclive.com/view/trastuzumab-deruxtecan-prolongs-pfs-in-pretreated-hr-her2-low-and--ultralow-metastatic-breast-cancer



