Drained Resources: Navigating the Nationwide IV Fluid Shortage and Its Toll on Oncology Care
Authors
- Ashley Jones, PharmD, BCOP, Clinical Specialist - Hematologic Malignancies, Hematopoietic Stem Cell Transplant & Cellular Therapies at UT Southwestern Medical Center, Dallas, TX
- Sanja Zepcan, PharmD, Clinical Specialist - Hematologic Malignancies, Hematopoietic Stem Cell Transplant & Cellular Therapies at Loyola University Medical Center, Chicago, IL
- Emily Rux, PharmD, BCOP, Clinical Specialist - Hematologic Malignancies, Hematopoietic Stem Cell Transplant & Cellular Therapies at Loyola University Medical Center, Chicago, IL
In late September 2024, Hurricane Helene devastated the southeast region of the U.S., forcing a Baxter Facility in North Carolina to close. Baxter’s plant, located in Marion, North Carolina, produces approximately 60% of the country’s intravenous (IV) fluid and dialysate solutions. This catastrophic event exacerbated an already existing nationwide shortage of IV fluids, significantly impacting healthcare delivery, particularly in oncology care. Current statistics reveal that the supply of IV fluids has dwindled to alarming levels, with many hospitals reporting a 50% reduction in available products1. The FDA conducted scientific and regulatory assessments to allow Baxter to begin importing 23 different IV and peritoneal dialysis (PD) fluids from sites around the world, with the goal of returning to 90-100% of the historical level of supply by the end of this year.
The question remains, though: What do we do in the meantime, and how do we navigate this critical shortage safely? As healthcare institutions deal with these challenges globally, the implications for patient care specifically in oncology settings are profound, highlighting the urgent need to ensure consistent access to essential medical supplies. Years before Hurricane Helene, medical facilities have experienced shortages of IV fluids, as reported by the FDA. Saline solution and sterile water have been on and off shortage since 2021, while several dextrose containing solutions have been in short supply since early 20222,3. These shortages have been exacerbated by Hurricane Helene's damage.
Premier Inc. conducted a survey on October 7th and 8th to evaluate IV fluid supplies in which 228 centers participated. This survey found that more than 86% of U.S. healthcare providers are experiencing shortages of IV fluids in the aftermath of recent hurricanes4. It is also reported that this has led to the cancellation of elective procedures and adoption of conservative approaches to IV fluid use and alternative strategies to preserve the remaining supply. In the survey, 54% of respondents reported having 10 days or less of IV fluids. Most hospitals have a reduced supply on hand, with limited supply available to replenish stock.
The shortage of fluids has huge implications for health systems as a whole. One of the major concerns being that many IV medications require dilution in IV piggybacks of dextrose or normal saline for administration. There are also many patient populations, such as the critically ill, that may require more urgent need of IV fluids and potentially may be prioritized in the setting of an extreme shortage. The shortage also has unique implications for our hematology, oncology, and bone marrow transplant patients as IV fluids are often administered to prevent toxicities of chemotherapy. Some common examples include continuous fluids for renal protection when administering nephrotoxic medications, such as cisplatin, continuous sodium bicarbonate infusions for urinary alkalization for high dose methotrexate, and prevention of hemorrhagic cystitis for cyclophosphamide and ifosfamide. Many oncologic emergencies, such as tumor lysis syndrome and hypercalcemia of malignancy require aggressive hydration as initial treatment with many guidelines recommending up to 3000 mL/m2/day to maintain adequate urine output.
Additionally, one of the most common side effects chemotherapy patients experience is nausea and vomiting, which frequently requires IV fluid support both in the inpatient and outpatient settings. Bone marrow transplant patients are standardly started on continuous fluids to prevent adverse events related to conditioning chemotherapy, to provide intravascular support throughout treatment, and to provide nutrition support in cases of mucositis or nausea and vomiting. The possibility of not being able to provide consistent fluids for our patient population has detrimental effects on the ability to support oncology patients or provide adequate treatment for them.
The below table presents a variety of suggested strategies aimed at conserving IV fluid supply specifically for oncology patients, ensuring both safe and effective treatment and resource management. Healthcare workers should use professional judgment in adopting the below information and should always consider the needs and resources of your individual organization(s).
Table 1: Suggested Strategies to Conserve IV Fluid Supply in Oncology Patients5,6,7
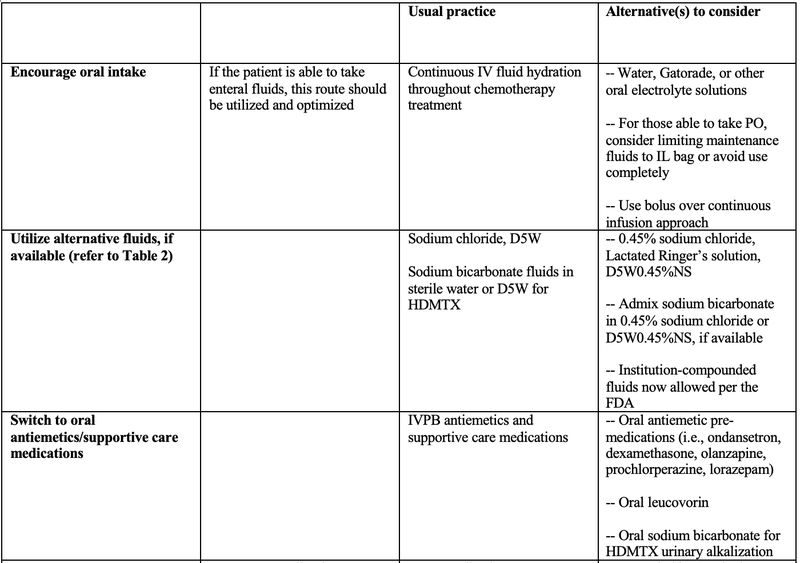
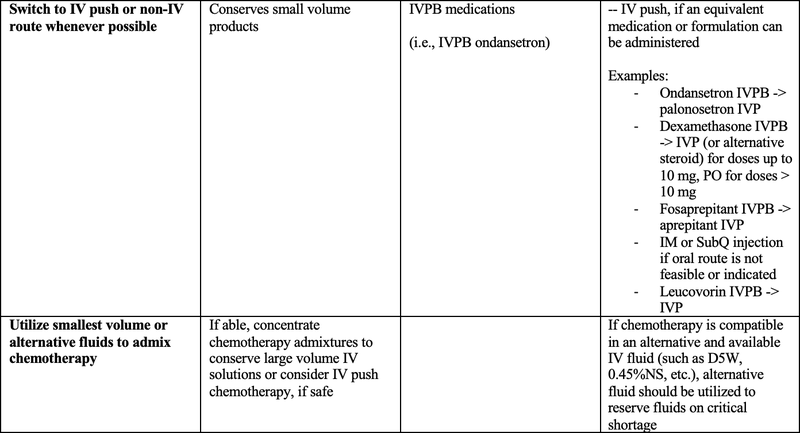
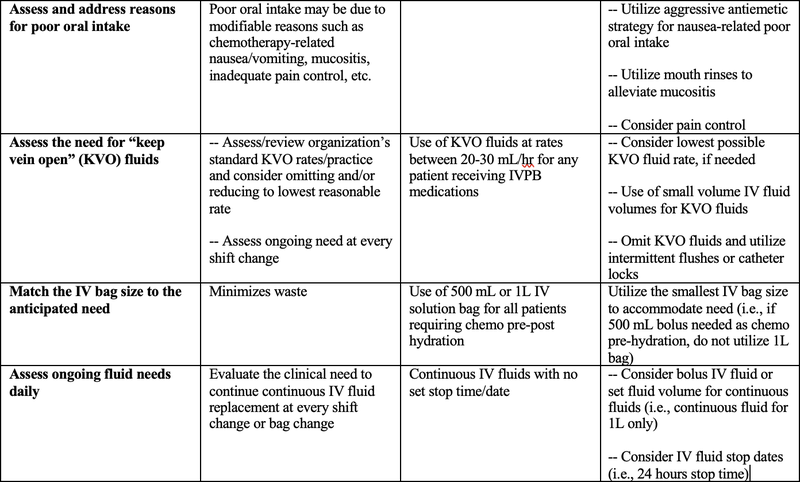
- IVPB = IV piggyback; IVP = IV push; SubQ = subcutaneous; PO = by mouth; FDA = U.S. Food and Drug Administration; HDMTX = high dose methotrexate
Table 2 (Adapted from ASHP Suggestions for management and conservation of IV fluids5,8,9,10,11,12,13)
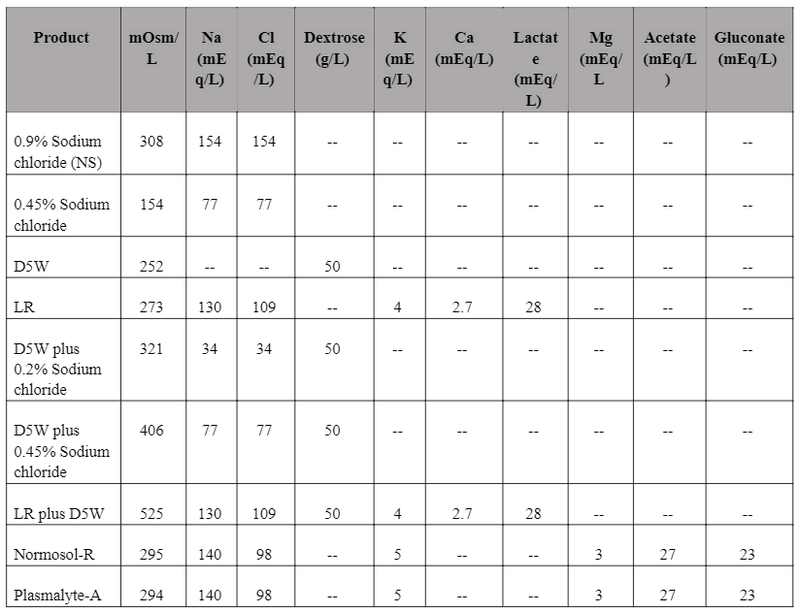
- NS - Normal Saline; D5W - Dextrose 5% in water; LR - Lactated Ringer's solution
As frustrating as the current shortage has been for all members of the healthcare team, there may be some positives that come from disaster conservation strategies. For example, in the future we may reduce overall waste by better delineating which oncology patients require continuous IV fluids or even KVO fluids, or by utilizing IV push and oral medications more often. Additionally, we may have a longer time period to utilize IV fluids due to extended expiration dates, which will reduce overall waste. In late October, after a review of stability data submitted by Baxter international, the FDA announced extended use dates for many parenteral drug products including D5W, sterile water, D5W0.9%NS, 0.45% NS, 0.9%NS, Lactated Ringer’s, and Plasmalyte1.
The IV fluid shortage has created significant challenges for healthcare facilities across the country, and we are hopeful for a resolution soon. The American Society of Health-System Pharmacists (ASHP) anticipates that the current IV fluid shortage may persist for several months14, and unfortunately, this is not the first time we have experienced a shortage of this magnitude (and it certainly will not be the last).
In order to improve the security of pharmaceutical supply chains of IV fluids and protect against inevitable future disaster scenarios, ASHP is urging government agencies to create a standing set of potential importation plans and consider delineating prioritization plans for critical medications15. One of these strategies may include diversification of suppliers to reduce reliance upon single source suppliers, as was the case in the current circumstance, where 60% of the country’s IV fluid supply came from the North Carolina Baxter facility. This action may ensure a more stable supply chain, even during disaster scenarios. Healthcare facilities may also consider completing a systematic utilization review and establishing guidelines for prioritization of IV fluids, which would allow for smoother allocation of IV fluids to those who need it the most in the future.
Ultimately, proactive measures and strategic planning are essential steps to safeguarding patient care and enhancing the resilience of the healthcare system in the face of future supply chain disruptions.
For the most up-to-date information on parenteral drug products currently in shortage as well as extended expiration dates, please visit the FDA website (updated in real time) 3
For the most up-to-date information on conservation strategies, please visit ASHP’s website (updated in real time)5
References
1.U.S. Department of Health and Human Services. Hurricane Helene: Baxter’s manufacturing recovery in North Carolina | FDA. Published online October 18, 2024. https://www.hhs.gov/about/news/2024/10/18/fact-sheet-hhs-continues-action-increase-access-supply-iv-fluids-hurricane-helene.html
2.Kantor, M. Long After Helene, IV Fluid Shortages Plague Hospitals. NBC News. https://www.nbcnews.com/health/health-news/long-helene-iv-fluid-shortages-plagued-hospitals-rcna175563. October 20, 2024.
3.Drug Shortage. Accessed October 21, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/drug-shortages.
4.Premier Inc. More than 86 Percent of Providers Experiencing Shortages of IV Fluids in Aftermath of October Hurricane. Accessed October 21, 2024. https://www.premierinc.com/newsroom/blog/premier-inc-data-more-than-86-percent-of-providers-experiencing-shortages-of-iv-fluids-in-aftermath-of-october-hurricane.
5.Small and large-volume fluid shortages – suggestions for management and conservation. Accessed October 23, 2024. https://www.ashp.org/drug-shortages/shortage-resources/publications/fluid-shortages-suggestions-for-management-and-conservation?loginreturnUrl=SSOCheckOnly
6.Patiño AM, Marsh RH, Nilles EJ, Baugh CW, Rouhani SA, Kayden S. Facing the Shortage of IV Fluids — A Hospital-Based Oral Rehydration Strategy. N Engl J Med. 2018;378(16):1475-1477. doi:10.1056/NEJMp1801772
7.Paquet F, Marchionni C. What Is Your KVO? Historical Perspectives, Review of Evidence, and a Survey About an Often Overlooked Nursing Practice. J Infus Nurs. 2016;39(1):32-37. doi:10.1097/NAN.0000000000000147
8.Lactated Ringer’s Injection, USP, [product information].
9.Lactated Ringer’s and Dextrose Injection, USP [product information]. Published online Deerfield, IL: Baxter 2019.
10.Dextrose and Sodium Chloride Injection, USP [product information]. Published online Deerfield, IL: Baxter 2019.
11.Sodium Chloride Injection, USP [product information]. Published online Deerfield, IL: Baxter 2024.
12.Dextrose Injection, USP [product information]. Published online Deerfield, IL: Baxter 2020.
13.Normosol-R pH 7.4 [product information]. Published online Lake Forest, IL: ICU Medical 2022.
14.ASHP urges federal agencies to respond to IV fluid shortages. Published online October 8, 2024. https://news.ashp.org/News/ashp-news/2024/10/08/ashp-urges-federal-agencies-to-respond-to-iv-fluid-shortages.
15.Tom Kraus, ASHP. IV solutions Shortages Recommendations; 2024. Accessed October 23, 2024. https://news.ashp.org/-/media/assets/advocacy-issues/docs/2024/ASHP-IV-Solutions-Shortage-Letter.pdf.



