FDA approves axatilimab-csfr for chronic graft-versus-host disease
What is the potential role for axatilimab-csfr in the treatment of chronic graft versus host disease (cGVHD)?
Axatilimab-csfr (NiktimvoTM) is a first-in-class colony stimulating factor-1 receptor (CSF-1R) blocker that binds to CSF-1 receptors on monocytes and macrophages, resulting in a decrease in the proinflammatory and profibrotic monocyte and macrophage driven effects that contribute to cGVHD.1
Axatilimab-csfr is FDA approved as a treatment option for adult and pediatric patients weighing at least 40 kg with cGVHD who have failed at least two prior lines of systemic therapy.1
Approval was based on the AGAVE-201 trial, a phase II, randomized, open-label, multicenter study.1,2
Methods
- AGAVE-201 included 241 adult and pediatric patients with active cGVHD who had previously received at least two prior lines of systemic therapy2
- Patients were randomly assigned (1:1:1) to receive axatilimab-csfr 0.3 mg/kg every 2 weeks, 1 mg/kg every 2 weeks, or 3 mg/kg every 4 weeks2
- Concomitant use of corticosteroids, calcineurin inhibitors, or mammalian target of rapamycin inhibitors was allowed2
- The primary endpoint was overall response rate (ORR), complete or partial, in the first 6 cycles (24 weeks) as defined by the National Institute of Health (NIH) 2014 Consensus criteria. The key secondary endpoint was the proportion of patients with a reported clinically significant reduction (> 5 points) in symptoms, measured by the modified Lee Symptom Scale (mLSS)2
Results
- Most patients included in the study had severe cGVHD (80%) and multi-organ involvement, with a median number of organs involved of 42
- Patients were heavily pretreated with a median of 4 prior lines of therapy which included ruxolitinib (74%), ibrutinib (31%), and belumosudil (23%)2
- A comparison of the three studied dosing strategies (0.3 mg/kg every 2 weeks, 1 mg/kg every 2 weeks, and 3 mg/kg every 4 weeks) revealed:2
- ORR of 74%, 67%, and 50%, respectively
- Adverse events leading to discontinuation in 6%, 22%, and 18% of the patients in the three dosing strategies, and fatal adverse events in 1%, 9%, and 8% of the patients respectively
- 0.3 mg/kg every 2 weeks had the highest ORR and least toxicity
- At the FDA approved dose of 0.3 mg/kg every 2 weeks:1,2
- Median time to clinically meaningful response was 1.7 months
- 60% of those who initially responded to axatilimab-csfr maintained a response at 12 months
- A clinically significant reduction in symptoms was observed in 60% of patients
- Organ-specific responses were observed in all organs, including those that had the most fibrotic changes
- Response rate: lower gastrointestinal (89%), upper gastrointestinal (82%), esophagus (78%), joints and fascia (76%), mouth (52%), lungs (47%), liver (40%), eyes (30%), and skin (26%)
- Adverse events that occurred in at least 10% of the patients in the 0.3 mg/kg every 2 weeks group included: any grade infection (73%), fatigue (23%), headache (19%), and diarrhea (16%)2
- Common laboratory abnormalities seen in the 0.3 mg/kg every 2 weeks group included: increased aspartate aminotransferase (AST) (14%), increased blood lactate dehydrogenase (14%), increased alanine aminotransferase (ALT) (13%), increased creatine kinase (11%), increased lipase (11%), and increased gamma glutamyl transferase (10%)2
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Hematopoietic Cell Transplantation were recently updated to include axatilimab-csfr as a category 2A recommendation for steroid-refractory cGVHD.3 Other FDA approved systemic agents for steroid-refractory cGVHD include ruxolitinib (category 1), ibrutinib, and belumosudil.3 Ruxolitinib is currently approved for cGVHD in patients ≥ 12 years of age after failure of 1 or 2 lines of systemic therapy, ibrutinib for patients ≥ 1 year of age after 1 or more lines of systemic therapy, and belumosudil for patients ≥ 12 years of age after failure of ≥ 2 prior lines of systemic therapy.4,5,6
Currently, there is an ongoing phase II trial evaluating axatilimab-csfr in combination with ruxolitinib for newly diagnosed cGVHD patients as well as a phase III trial from Japan looking to further evaluate axatilimab-csfr’s efficacy, safety, and pharmacokinetics in recurrent or refractory cGVHD.7,8 No randomized data is currently available comparing axatilimab-csfr to other agents utilized in steroid-refractory cGVHD.3
What role can the pharmacist play in the management of patients on axatilimab-csfr?
Dosing1,2
- The labeled dosing for axatilimab-csfr is 0.3 mg/kg (max 35 mg) every 2 weeks administered as an IV infusion over 30 minutes
- Based on the AGAVE-201 study, patients that achieved a sustained partial or complete response for > 20 weeks or did not progress could transition to 0.6 mg/kg every 4 weeks. If a patient progressed following the dose change, they could return to every 2-week dosing
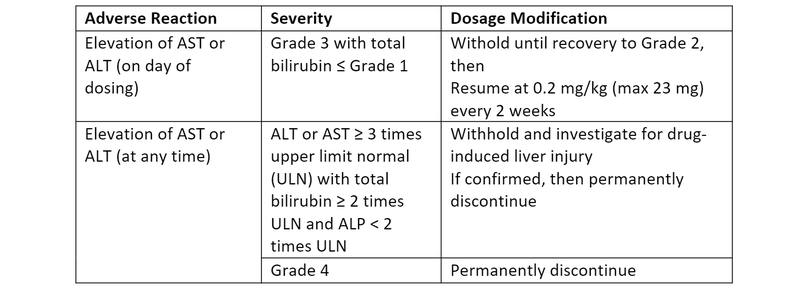
Dosage Modifications for AST and ALT Elevations1
Infusion-Related Reactions1
- Infusion-related reactions occurred in 8% of patients receiving 0.3 mg/kg every 2 weeks in the AGAVE-201 trial
- Management of infusion-related reactions includes slowing the rate of the infusion by 50% and administering antipyretics and antihistamines for Grade 1 and 2 reactions. Axatilimab-csfr should be permanently discontinued for Grade 3 or 4 reactions
- For patients who have previously experienced a Grade 1 or 2 reaction to axatilimab-csfr, it is recommended to premedicate with an antihistamine or antipyretic for future doses
Patient Assistance Program
- At this time, there is no published information on patient assistance programs available for axatilimab-csfr
Clinical Pearls1
- Axatilimab-csfr is currently supplied as a single-dose 50 mg/mL vial. Incyte is seeking approval of two smaller vial sizes to reduce product waste given the max dose is 35 mg
- Store vials refrigerated at 2◦C to 8◦C (36◦F to 46◦F) in the original carton to protect from light. Do not shake or freeze
- Doses for infusion are prepared by aseptically combining axatilimab-csfr with 0.9% sodium chloride to achieve a final concentration within the range of 0.24 mg/mL to 0.75 mg/mL
- Infusions are administered over 30 minutes through a dedicated line with either a 0.2 micron in-line filter or an add-on polyethersulfone (PES) filter
- Axatilimab-csfr has a short beyond use dating once diluted. At room temperature, the infusion must be completely administered within 4 hours of its preparation or may be refrigerated for up to 24 hours
- Monitor patients for infusion-related reactions. If infusion-related reactions occur, treat with antipyretics and antihistamines and slow the infusion rate by 50%
- Verify pregnancy status prior to treatment initiation. Patients who could become pregnant should use an effective form of birth control during axatilimab-csfr treatment and for 30 days following their last dose
References
1.Niktimvo (axatilimab-csfr) [package insert]. Wilmington, DE: Incyte Corporation; August 2024
2.Wolff D, Cutler C, Lee SJ, et al. Axatilimab in Recurrent or Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2024;391(11):1002-1014. doi:10.1056/NEJMoa2401537
3.National Comprehensive Cancer Network. NCCN Guidelines Version 1.2024 Hematopoietic Cell Transplantation. Accessed September 30, 2024.
4.Jakafi (ruxolitinib) [package insert]. Wilmington, DE: Incyte Corporation; January 2023
5.Imbruvica (ibrutinib) [package insert]. Horsham, PA: Janssen Biotech Inc; February 2024
6.Rezurock (belumosudil) [package insert]. Bridgewater, NJ: Kadmon Pharmaceuticals; April 2024
7.A Study to Evaluate the Safety and Efficacy of Axatilimab in Combination with Ruxolitinib in Participants with Newly Diagnosed Chronic Graft-Versus-Host Disease. ClinicalTrials.gov identifier: NCT06388564. Updated August 5, 2024. Accessed September 4, 2024. https://clinicaltrials.gov/study/NCT06388564#study-record-dates
8.A Study to Evaluate the Efficacy, Safety, and Pharmacokinetics of Axatilimab Monotherapy in Japanese Participants with Recurrent or Refractory Active Chronic Graft-Versus-Host Disease. ClinicalTrials.gov identifier: NCT06263478. Updated August 16, 2024. Accessed September 4, 2024. https://clinicaltrials.gov/study/NCT06263478?intr=axatilimab&page=1&rank=7



