What is the potential role for mirdametinib in the treatment of neurofibromatosis type 1 in adult and pediatric patients with symptomatic plexiform neurofibromas not amendable to complete resection?
- Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder caused by germline mutations in the NF1 tumor suppressor gene on chromosome 17 which can impact many organ systems. Neurofibromas are non-malignant tumors that develop around nerve sheaths, which are a characteristic of NF1 and can often decrease patient quality of life. In some cases, neurofibromas involve larger nerves, which are called plexiform neurofibromas (PN). PN are often more diffuse tumors which can lead to pain, disfigurement, and potential risk of malignant transformation.1,2
- Clinical manifestations and symptom presentation vary in the pediatric and adult population. Characteristics of NF1 can include pigmentary changes, neurofibromas, PN, learning disabilities, skeletal abnormalities, and development of low-grade tumors and other malignancies.1
- Surgical management has historically been the primary treatment for PN, however surgical removal is often associated with life-changing morbidities and does not always lead to complete resection of the tumor.1,2
- Mirdametinib is the first and only FDA approved drug for the treatment of NF1-associated PN in both adult and pediatric patient populations when surgical management is not an option.
- Mirdametinib is a highly selective, potent, MEK1/2 inhibitor with CNS penetration.
- Approval was based on the ReNeu trial, a pivotal multicenter, phase IIb, open-label, single-arm study that evaluated mirdametinib in adult and pediatric patients with inoperable PN.2
- Methods
- Included 58 adults and 56 pediatric patients with NF1-PN causing significant morbidity. Adults and pediatrics were enrolled in separate cohorts.
- Patients were given mirdametinib 2 mg/m2 twice daily (max dose of 4 mg twice daily) on days 1-21 of a 28-day cycle for 24 cycles.
- The primary endpoint was confirmed objective response rate (ORR) which was defined as the proportion of patients with a ≥ 20% reduction on MRI of the target PN volume from baseline to cycle 24 on ≥ 2 consecutive scans within 2-6 months.
- Results
- In the adult cohort, 41% achieved confirmed ORR during the treatment phase which surpassed the 23% predefined minimum (p < 0.001).
- In the pediatric cohort, 52% achieved confirmed ORR during the treatment phase which surpassed the 20% predefined minimum (p < 0.001).
- Mirdametinib led to a deep and durable tumor volume reduction based on improvement in median percent change in target PN volume (adults: -41% [range, -90 to13]; pediatrics: -42% [range, -91 to 48]).
- Significant reductions were reported in worst tumor pain severity and pain interference in both cohorts as well as significant improvements in health-related quality of life.
- Methods
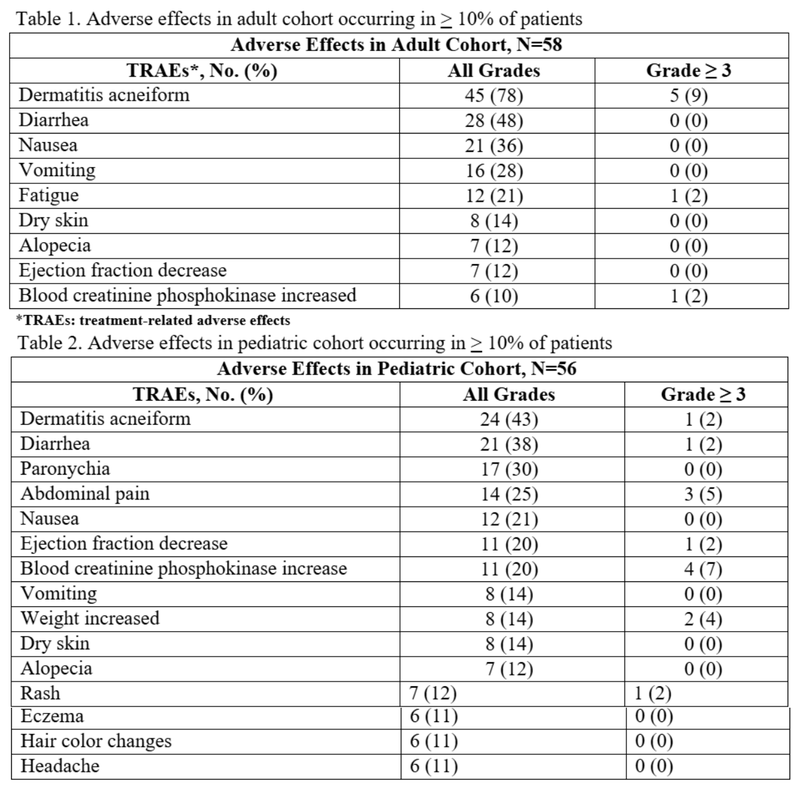
- Dose reductions were required in 17% of adult patients and 12% of pediatric patients due to TRAEs. Dose interruptions were experienced in 31% of adult patients and in 30% of pediatric patients.2 Twenty-two percent of adults and 9% of pediatric patients discontinued treatment due to adverse effects.
- Other MEK inhibitors have been used off-label in adult populations, such as selumetinib. Selumetinib is FDA approved for NF1-PN in patients ages 2-17. However, this agent is only formulated as a capsule which can be a barrier for individuals with dysphagia.2
What role can the pharmacist play in the management of patients on mirdametinib?
- Dosing
- Mirdametinib is taken on days 1-21 of a 28-day cycle, and the recommended dose is 2 mg/m2 twice daily with a maximum dose of 4 mg twice daily.
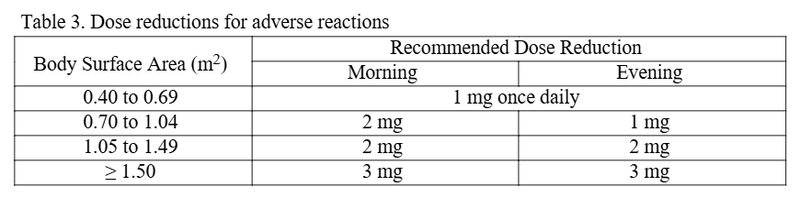
- Reassess dosing as the patients’ body surface area changes.
- Dose reductions are advised in the setting of adverse effects.
- No clinical drug interaction studies have been conducted. Mirdametinib is a substrate of BCRP and P-gp transporters. There are no renal or hepatic dose adjustments recommended.3
- Supportive Care for Gastrointestinal Reactions
- Patients can consider adopting the BRAT diet (bananas, rice, applesauce, and toast or plain pasta) and avoid foods that could exacerbate GI distress such as fried, fatty, or spicy foods.
- If noninfectious cause of diarrhea is determined, diarrhea may be managed with antidiarrheals.
- Management of Dermatologic Adverse Reactions
- Rash of any grade occurred in 90% of adult patients and 73% of pediatric patients in the ReNeu trial. However, ≥ grade 3 reactions occurred in 10% of adults and 4% of pediatric patients.
- Rashes can present various ways, but dermatitis acneiform was most common in ages 12-17 years, and non-acneiform rashes were most common in ages 2-11 years.
- Prophylactic treatment for postpubescent patients at the time of mirdametinib initiation may include topical clindamycin 1% lotion that can be applied to the face twice daily and a tetracycline such as doxycycline 50 mg daily for the first 3 months of treatment.
- Topical steroid creams or antibiotics may be added if mild-severe acneiform rash develops.4
- Ocular Changes May Occur
- Comprehensive ophthalmic assessments at time of treatment initiation and regularly throughout treatment is preferred.
- Monitor changes in vision, such as increased blurriness. Retinal vein occlusion and retinal pigment epithelium detachment were reported in the ReNeu trial.3
- Left Ventricular Dysfunction May Occur
- A decrease in left ventricular ejection fraction of 10% to <20% occurred in 16% of adults and 25% of pediatric patients. The median time to onset in adults was 70 days and 132 days in the pediatric population.
- Assess ejection fraction by echocardiogram prior to initiation and every 3 months during the first year of treatment. Then, as clinically indicated thereafter.
- Embryo-Fetal Toxicity
- Mirdametinib may cause fetal harm when administered to pregnant patients resulting in spontaneous abortion. Advise female patients of reproductive potential to use effective contraception during treatment and for 6 weeks after the last dose of mirdametinib. For male patients, it is advised to use effective contraception during treatment and for 3 months after the last dose of mirdametinib.3
- Patient Assistance through SpringWorks Care Connections
- SpringWorks Care Connections offers financial assistance for treatment-related expenses for eligible, commercially insured patients. These can cover cardiology visits and testing, dermatology visits, and ocular exams.
- Submitting a prior authorization or medical exception might be required for this medication, however, there is potential for short-term access to free drug to be delivered if this process delays treatment.5
Clinical Pearls
- Formulations
- Mirdametinib has two dosage form options: capsules and dispersible tablets that can be made into an oral suspension. Having a liquid option can be favorable for pediatric patients and those with dysphagia. The tablet comes as 1 mg, and the capsule comes as both 1 mg and 2 mg doses.3
- Tablets for oral suspension
- To prepare a tablet for oral suspension, add approximately 5 mL to 10 mL of drinking water into a dosing cup followed by the prescribed number of tablets. Swirl cup until complete dispersion which is about 2-4 minutes. Swallow medication within 30 minutes of compounding or dose will need to be re-prepared.
- To ensure all the medication was swallowed, refill dosing cup with approximately 5 mL to 10 mL of water. Swirl and drink again to ensure any remaining tablet residue is resuspended.
- To prepare a tablet for oral suspension, add approximately 5 mL to 10 mL of drinking water into a dosing cup followed by the prescribed number of tablets. Swirl cup until complete dispersion which is about 2-4 minutes. Swallow medication within 30 minutes of compounding or dose will need to be re-prepared.
- Capsules
- Swallow capsules one at a time if more than one capsule is required.3
- Mirdametinib can be taken with or without food.2-3
- Storage and handling
- Store capsules and tablets at room temperature and protect from light.3
References
1.Gutmann, D., Ferner, R., Listernick, R. et al. Neurofibromatosis type 1. Nat Rev Dis Primers 3, 17004 (2017). https://doi.org/10.1038/nrdp.2017.4.
2.Moertel CL, Hirbe AC, Shuhaiber HH, Bielamowicz K, Sidhu A, Viskochil D, et al. ReNeu: A Pivotal, Phase IIb Trial of Mirdametinib in Adults and Children With Symptomatic Neurofibromatosis Type 1-Associated Plexiform Neurofibroma. J Clin Oncol. 2025 Feb 20;43(6):716-729. doi: 10.1200/JCO.24.01034.
3.Gomekli (mirdametinib) [prescribing information]. Stamford, CT: SpringWorks Therapeutics,Inc; March 2025.
4.SpringWorks Therapeutics. 2025. Gomekli Dosing and Adverse Reaction Management Guide [Brochure]. SpringWorks Therapeutics, Inc. GOMEKLI (mirdametinib) Dosing and Adverse Reaction Management Guide.
5.SpringWorks Therapeutics. 2025. Gomekli Treatment Access Guide [Brochure]. SpringWorks Therapeutics, Inc. GOMEKLI (mirdametinib) Treatment Access Guide.




