Please note at the time of publication for this PAP, a link to this drug update on fda.gov was not available. From the manufacturer's website: Momelotinib approved in the US for treatment of myelofibrosis patients with anaemia.
What is the potential role for momelotinib in the treatment of myelofibrosis?
- Momelotinib works by inhibiting disease drivers such as JAK1 and JAK2, which contribute to the pathogenesis of myelofibrosis (MF). Unlike other JAK inhibitors, momelotinib also inhibits activin A receptor type 1 (ACVR1), which reduces liver hepcidin expression and increases iron availability for erythropoiesis.1,2
- Momelotinib received its FDA approval based on results of the Phase III MOMENTUM trial3:
- In the MOMENTUM trial, 195 symptomatic and anemic patients with MF who had previously taken an approved JAK inhibitor were randomized to receive either momelotinib 200 mg daily or danazol 300 mg twice daily for 24 weeks
- Sixty-four percent of patients had primary MF, 19% had post-polycythemia vera (PV) MF, and 17% had post-essential thrombocytopenia (ET) MF
- The primary endpoint was the Myelofibrosis Symptom Assessment Form (MFSAF) TSS response rate at 24 weeks which was defined as a ≥ 50% reduction in mean MFSAF TSS over the 28 days before the end of week 24 compared to baseline
- More patients in the momelotinib cohort reported a 50% or more reduction in TSS than in the danazol group (32 [25%] of 130 vs six [9%] of 65; proportion difference 16% [95% CI 6–26], p=0.0095)
- Additionally, patients in the momelotinib group reported a rapid increase in hemoglobin that was maintained longer than with danazol
- Momelotinib had a greater percentage of patients with transfusion independence between weeks 12 and 24 compared to danazol (30% vs 20%; 0.0116 [one-sided; non-inferior])
- More patients experienced a splenic response rate, defined as >25% reduction, in the momelotinib group than in the danazol group (39% vs 6%, p < 0.0001)
- Most frequently reported grade 3 or higher adverse events of momelotinib and danazol, respectively, includes anemia (61% vs 75%), thrombocytopenia (28% vs 26%), acute kidney injury (3% vs 9%), and pneumonia (2% vs 9%).
- Three cardinal features of myelofibrosis include anemia (Hb < 10 g/dL), splenomegaly, and constitutional symptoms1
- Anemia is an adverse prognostic factor in myelofibrosis that is an unmet clinical need with other JAK2 inhibitors1
- Ruxolotinib and Fedratinib were found to accentuate anemia in the COMFORT-II trial and JAKARTA2 trial, respectively; therefore, these products are a poor option for severely anemic patients1,5,6
- Momelotinib was previously found to be non-inferior to ruxolitinib in the front-line setting in regards to reducing spleen volume, improvements in transfusion burden, and symptom reduction in the SIMPLIFY-1 trial1,7
- However, momelotinib did not demonstrate superiority in providing additional spleen volume reductions in SIMPLIFY-2 trial1,8
- Due to the promising data in symptom resolution in comparison to danazol and increases in hemoglobin in MOMENTUM, momelotinib is positioned to address patients with severe anemia
What role can the pharmacist play in the management of patients on momelotinib?
- Pharmacists play an integral role in ensuring the correct dose of momelotinib and monitoring for subsequent dose adjustments.
- The starting dose of momelotinib is 200 mg by mouth once daily2
- Patients with severe hepatic impairment should have their initial dose reduced to 150 mg once daily2
- Pharmacists can optimize dose modifications in relation to thrombocytopenia, neutropenia, hepatotoxicity, and other grade 3 or higher events per the package insert
Clinical pearls:
- Momelotinib is an OATP1B1/B3 substrate and breast cancer resistance protein (BCRP) inhibitor
- Rosuvastatin (a BCRP substate) when used concomitantly with momelotinib should be initiated at 5 mg and not increased to more than 10 mg once daily2
- Risk of Hepatitis B reactivation with or without associated elevations in alanine transaminase (ALT) or aspartate transaminase (AST)2
References:
1.Chifotides HT, Bose P, Verstovsek S. Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J Hematol Oncol. 2022;15(1):7. doi:10.1186/s13045-021-01157-4
2.OJJAARA (momelotinib). Prescribing Information. GSK; 2023.
3.Verstovsek S, Gerds AT, Vannucchi AM, et al; MOMENTUM Study Investigators. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis (MOMENTUM): results from an international, double-blind, randomised, controlled, phase 3 study. Lancet. 2023;401(10373):269-280. doi:10.1016/S0140-6736(22)02036-0
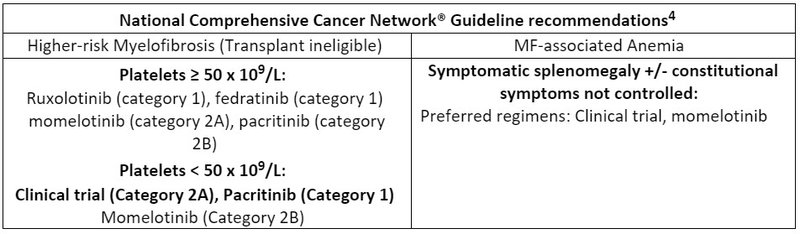
4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Myeloproliferative Neoplasms V.1.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. Accessed January 5th, 2024. To view the most recent and complete version of the guideline, go online to NCCN.org.
5.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis [published correction appears in Leukemia. 2017 Mar;31(3):775]. Leukemia. 2016;30(8):1701-1707. doi:10.1038/leu.2016.148
6.Harrison CN, Schaap N, Vannucchi AM, et al. Fedratinib in patients with myelofibrosis previously treated with ruxolitinib: An updated analysis of the JAKARTA2 study using stringent criteria for ruxolitinib failure. Am J Hematol. 2020;95(6):594-603. doi:10.1002/ajh.25777
7.Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: A Phase III Randomized Trial of Momelotinib Versus Ruxolitinib in Janus Kinase Inhibitor-Naïve Patients With Myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
8.Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73-e81. doi:10.1016/S2352-3026(17)30237-5