FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer
What is the potential role for Sacituzumab govitecan-hziy in the treatment of breast cancer?
- Sacituzumab govitecan-hziy is an antibody-drug conjugate (ADC) that combines a humanized monoclonal antibody targeting Trop-2 with SN-38. Once bonded to Trop-2 on cancer cells, the ADC is internalized, and the SN-38 component is released. This leads to DNA damage and cell death through apoptosis in both the tumor cells and the surrounding tumor microenvironment.1
- Sacituzumab govitecan-hziy is recommended in the National Comprehensive Cancer Network (NCCN) guidelines as second-line therapy for recurrent unresectable or metastatic, HR-positive/HER2-negative or triple-negative breast cancer.2
- Approval for the HR-positive/HER2-negative indication was based on the TROPiCS-02 Trial 3,4
- 543 patients were randomized in this open label, multicenter phase III trial that compared sacituzumab govitecan-hziy (n=272) to single agent chemotherapy [eribulin (n=130), vinorelbine (n=63), gemcitabine (n=56), or capecitabine (n=22)].
- Patients were eligible if they had unresectable, locally advanced or metastatic HR-positive/HER2-negative breast cancer whose disease progressed after a CDK 4/6 inhibitor, endocrine therapy, and a taxane. Patients received at least two prior chemotherapies in the metastatic setting.
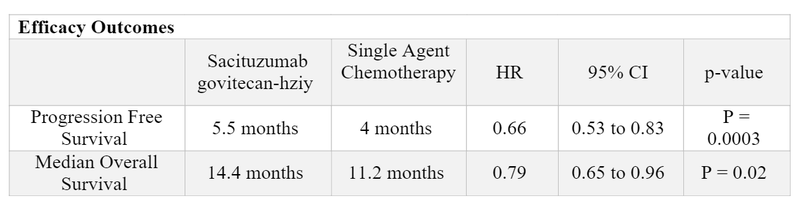
- The most frequent treatment-emergent adverse events of any grade were neutropenia (71%), diarrhea (62%), nausea (59%), alopecia (48%), and anemia (37%). The most common grade > 3 treatment-emergent adverse events were neutropenia (51%) and diarrhea (10%).
- Approval for triple-negative breast cancer (TNBC) was based on the ASCENT trial 5,6,7
- There were 529 patients randomized in this open label, multicenter, phase III trial that compared sacituzumab govitecan-hziy (n=267) to single agent chemotherapy [eribulin (n=139), vinorelbine (n=52), gemcitabine (n=38), or capecitabine (n=33)].
- Patients were eligible if they had unresectable locally advanced or metastatic TNBC who had relapsed after at least two prior chemotherapies, one of which could be in the neoadjuvant or adjuvant setting if progression occurred within 12 months.
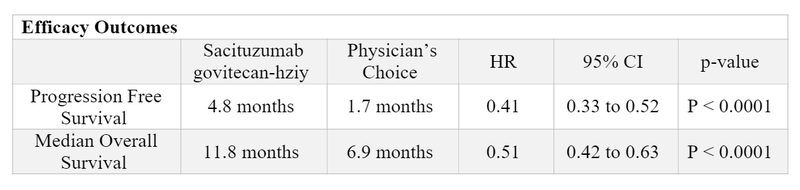
- Most common treatment-related adverse reactions included: neutropenia (63%), diarrhea (59%), nausea (57%), alopecia (46%), fatigue (45%), and anemia (34%). The most common grade > 3 treatment-related adverse events were neutropenia (51%), leukopenia (10%), diarrhea (10%), anemia (8%), and febrile neutropenia (6%).
- Other treatment options used in third-line setting include:2
- Fam-trastuzumab deruxtecan-nxki, however patients must be HER2 IHC 1+ or 2+/ISH negative
- Systemic chemotherapy such as doxorubicin, paclitaxel, capecitabine, gemcitabine, and vinorelbine; however, chemotherapy is frequently discontinued because of unacceptable toxicity.
- Note: NCCN lists as second-line setting. For early recurrences, these options can be second-line but for late recurrences it’s technically 3rd per the trial design.
What role can the pharmacist play in the management of patients on sacituzumab govitecan-hziy?
- Pharmacists have a large role in ensuring patients on sacituzumab govitecan-hziy are dosed appropriately, monitoring for adverse events, and helping prevent and manage adverse events when they do occur.
- Dosing and Administration
- The recommended dosage of sacituzumab govitecan-hziy is 10 mg/kg administered as an intravenous infusion once weekly on days 1 and 8 of 21-day treatment cycles.
- It is recommended the first infusion be administered over 3 hours, followed by an observation period for at least 30 minutes. In subsequent cycles, the infusion can be administered over 1 to 2 hours if previous infusions were tolerated.
- Patients should be premedicated with antipyretics, H1 and H2 blockers prior to infusion, and corticosteroids to prevent infusion reactions, and a two to three drug regimen is recommended (for example, with dexamethasone and a 5-HT3 receptor antagonist or an NK1 receptor antagonist to prevent chemotherapy-induced nausea and vomiting.
- There are no renal or hepatic dose adjustments.
- Sacituzumab govitecan-hziy is a substrate of UGT1A1 and concomitant use of UGT1A1 inhibitors can increase systemic exposure to SN-38, and UGT1A1 inducers may decrease exposure to SN-38. Additionally, patients with a reduced UGT1A1 activity are at an increased risk for neutropenia, febrile neutropenia, and anemia and may be at increased risk for other adverse reactions when treated with sacituzumab govitecan-hziy.
- Adverse events and management information:
- The most prevalent adverse reactions include diarrhea (64%), nausea (64%), fatigue (51%), alopecia (45%), constipation (37%), vomiting (35%), and decreased appetite (30%).
- The most prevalent laboratory abnormalities include decreased leukocyte count (84%), decreased neutrophil count (75%), decreased hemoglobin (69%), decreased lymphocyte count (63%), increased glucose (37%), decreased albumin (35%), decreased creatinine clearance (28%), increased alkaline phosphatase (28%), decreased magnesium (27%), decreased potassium (26%), and decreased sodium (26%).
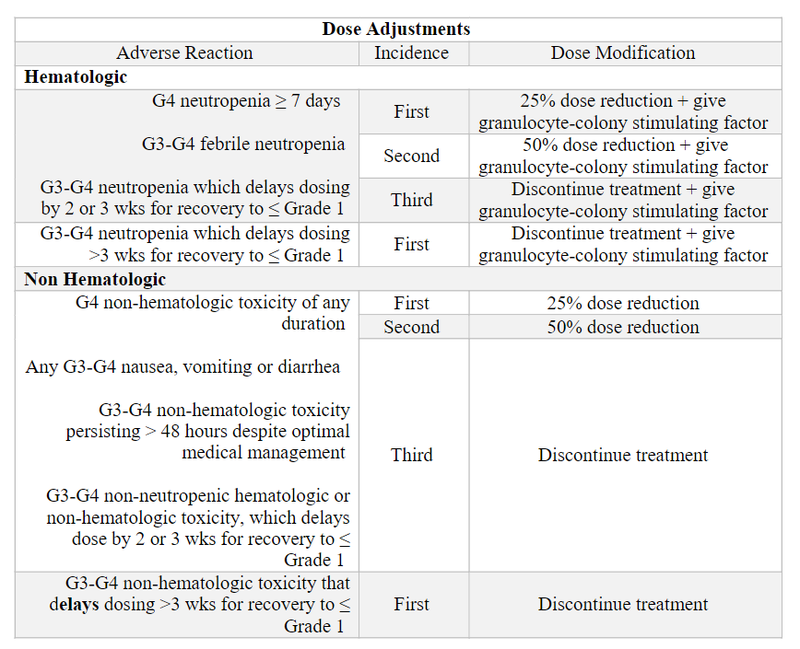
- Should patients develop G3 or G4 hematologic or non-hematologic adverse events, the current dose should be reduced or discontinued. Doses should not be re-escalated following a dose reduction.
- Patients should regularly have blood counts monitored, given severe neutropenia and neutropenic fever can occur.
- Per the NCCN gudelines, sacituzumab govitecan-hziy is a regimen with an intermediate risk for febrile neutropenia; therefore, patients should be closely evaluated for risk factors (e.g., age >65, bone marrow involvement of tumor, prior chemotherapy/ radiation therapy) and considered for prophylactic use of granulocyte-colony stimulating factor.8
- Sacituzumab govitecan-hziy is a regimen with a high emetic risk. Patients should receive premedication prevention of chemotherapy-induced nausea and vomiting, and for prevention of infusion reactions.
- Patient assistance programs:
- Commercial or private insurance: co-pay program that offers up to $25,000 annually for out-of-pocket expenses of sacituzumab govitecan-hziy 180-mg single-dose vials.
- Government: assistance may be available through an independent co-pay assistance foundation. Program specialists are available to help patients assess their eligibility.
- Uninsured: patients who are uninsured may be eligible to obtain sacituzumab govitecan-hziy at no cost through the Gilead Patient Assistance Program. Patients must be enrolled in Gilead Oncology Support.
Clinical Pearls
- Sacituzumab govitecan-hziy should be protected from light, including during administration. It is not necessary to cover the infusion tubing or to use light-protective tubing during the infusion.
- If not used immediately, sacituzumab govitecan-hziy can be stored refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours protected from light. After refrigeration, administer diluted solution at room temperature up to 25°C (77°F) within 8 hours (including infusion time).
- In the ASCENT trial, 12% of patients had baseline brain metastases previously treated and stable (n=61; 32 on sacituzumab govitecan-hziy arm and 29 on single agent chemotherapy arm. The PFS in patients with previously treated, stable brain metastases showed a stratified HR of 0.65 (95% CI: 0.35, 1.22). The median PFS in the sacituzumab govitecan-hziy arm was 2.8 months vs. 1.6 months in the single chemotherapy arm.
- A post hoc analysis of ASCENT evaluated efficacy outcomes in patients who required dose interruption or dose reduction, compared with the overall population (only patients without brain metastasis were included in the post-hoc analysis). Regardless of dose modification, patients receiving sacituzumab govitecan-hziy had improved outcomes versus patients receiving chemotherapy, and patients in the sacituzmab govitecan-hziy group who had dose interruptions or reductions had similar efficacy outcomes compared with patients without dose modifications.9
- PRIMED, a phase II clinical trial, assessed the feasibility of primary prophylaxis with G-CSF (300 mcg for 2 days) and loperamide (2 mg twice daily or 4 mg daily for 3 days) after each dose during the first two cycles of sacituzumab govitecan-hziy to improve the tolerability of sacituzumab govitecan-hziy and decrease treatment modifications.10 During the first two cycles, the incidence of any grade neutropenia and diarrhea were 28% and 34%, respectively. Additionally, rates of adverse events leading to dose reductions and dose interruptions were 14% and 30% respectively. Both the overall incidence of diarrhea and neutropenia as well as the frequency of dose reductions were lower in PRIMED than in ASCENT or TROPiCS02. This approach could help mitigate dose modifications and potentially prevent treatment discontinuations. The pharmacist should consider the feasibility of short-acting G-CSF after each dose of sacituzumab govitecan-hziy to improve neutrophil counts on day 8 compared to long-acting G-CSF after day 8 only.
References
1.Trodelvy (sacituzumab govitecan) [prescribing information]. Foster City, CA: Gilead Sciences Inc; February 2023.
2.National Comprehensive Cancer Network. Breast Cancer (Version 6.2024). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed November 21, 2024.
3.U.S. Food and Drug Administration. FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer. Febuary 2023. Accessed November 21,2024.
4.Rugo HS, Bardia A, Marmé F, et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023;402(10411):1423-1433. doi:10.1016/S0140-6736(23)01245-X
5.U.S. Food and Drug Administration. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. April 2021. Accessed November 21,2024
6.Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2021;384(16):1529-1541. doi:10.1056/NEJMoa2028485
7.Bardia A, Rugo HS, Tolaney SM, et al. Final Results From the Randomized Phase III ASCENT Clinical Trial in Metastatic Triple-Negative Breast Cancer and Association of Outcomes by Human Epidermal Growth Factor Receptor 2 and Trophoblast Cell Surface Antigen 2 Expression J Clin Oncol. 2024;42(15):1738-1744.
8.National Comprehensive Cancer Network. Hematopoietic Growth Factors. (Version 1.2025). https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf. Accessed November 21, 2024
9.Rugo HS, Tolaney SM, Loirat D, et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. npj Breast Cancer. 2022;98(8).
10.Pérez García JM, Gión M, Ruiz-Borrego M. Prevention of sacituzumab govitecan (SG)-related neutropenia and diarrhea in patients (pts) with triple-negative or HR+/HER2- advanced breast cancer (ABC; PRIMED): A phase 2 trial. J Clin Oncol 2024;42(16):suppl 1101.




