What is the potential role for vorasidenib in the treatment of astrocytoma or oligodendroglioma?
- Vorasidenib is an isocitrate dehydrogenase-1 and -2 (IDH1 and IDH2) inhibitor.1
- Vorasidenib is approved for treatment of patients with grade 2 astrocytoma or oligodendroglioma with IDH1 or IDH2 mutations.1
- IDH mutations in gliomas produce an oncometabolite D-2-hydroxyglutarate (D-2-HG) which stimulates tumor growth. IDH inhibitors work by decreasing the D-2-HG production to suppress tumor growth and promote cellular differentiation.2
- Vorasidenib was approved by the FDA based on the INDIGO trial, a double-blind, placebo-controlled, international, phase 3 trial, in adults with residual or recurrent grade 2 IDH-mutated gliomas.3
- Patients received vorasidenib 40 mg daily (n=168) or placebo (n=163) on days 1 through 28 in 28-day cycles.
- The imaging-based progression free survival (PFS) was higher in the vorasidenib group compared to the placebo group (27.7 months vs. 11.1 months; HR 0.39; 95% CI 0.27-0.56; p = <0.001).
- Time to next intervention was longer in the vorasidenib group compared to the placebo group (hazard ratio, 0.26; 95% CI, 0.15 to 0.43; P<0.001)
- Adverse events leading to dose reductions occurred in 18 (10.8%) patients, dose interruptions occurred in 50 (29.9%) patients, and discontinuation occurred in 6 (3.6%) patients.
- The most common reason for dose adjustments was alanine transaminase (ALT) elevation, for discontinuation was ALT elevation, and for interruption was ALT and/or aspartate transferase (AST) elevation and COVID-19 infection.
- The most common adverse events seen were fatigue (37%), COVID-19 (33%), musculoskeletal pain (26%), diarrhea (25%), and seizure (16%); the most common serious adverse were increased aminotransferase level (4.2%) and an increased γ-glutamyltransferase (GGT) level (3.0%).
- Currently, vorasidenib is the first targeted therapy approved for adjuvant treatment in gliomas.
- Other IDH inhibitors include ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor). Ivosidenib and enasidenib are potent IDH1 and IDH2 inhibitors, respectively, but they have low penetration of the blood brain barrier.4 Vorasidenib is more effective in penetrating the blood brain barrier.5
- To date, ivosidenib has been included in glioma treatment guidelines for use in certain circumstances, but it is not FDA approved for treatment of patients with IDH-mutated astrocytomas or oligodendrogliomas. Enasidenib currently has no role in the treatment of gliomas.
- The National Comprehensive Cancer Network (NCCN) guidelines include vorasidenib as a treatment option in the following scenarios:
- For astrocytoma:
- Preferred adjuvant treatment of patients with WHO grade 2 IDH-mutant astrocytoma after surgery/biopsy and treatment with radiation and chemotherapy is not preferred, and Karnofsky Performance Status (KPS) of ≥60.
- Adjuvant treatment of patients with WHO grade 2 IDH-mutant astrocytoma and a KPS <60.
- Preferred treatment of patients with recurrent or progressive WHO grade 2 IDH-mutant astrocytoma after treatment with radiation and chemotherapy of, and a KPS of ≥60.
- Preferred treatment of patients with recurrent or progressive WHO grade 3 or 4 IDH-mutant astrocytoma and a KPS of ≥60.4
- For Oligodendroglioma:
- Preferred adjuvant treatment of patients with WHO grade 2 IDH-mutant oligodendroglioma after surgery/biopsy and treatment with radiation and chemotherapy is not preferred, and KPS of ≥60.
- Adjuvant treatment of patients with WHO grade 2 IDH-mutant oligodendroglioma and a KPS <60.4
- Preferred treatment of patients with recurrent or progressive WHO grade 2 IDH-mutant oligodendroglioma after radiation and chemotherapy and KPS of ≥60.
- Preferred treatment of patients with recurrent or progressive WHO Grade 3 IDH-mutant oligodendroglioma and a KPS of ≥60.4
- For astrocytoma:
What role can the pharmacist play in the management of patients taking vorasidenib?
- Pharmacists can have a role in vorasidenib management including, but not limited to, dosing, drug interaction identification and monitoring, lab monitoring, adverse effect management, and dose adjustments due to toxicity.
- Dosing for adults is 40 mg orally daily.
- Dosing for pediatric patients aged 12 years and older is based on weight. For patients weighing less than 40 kg, the dose is 20 mg by mouth daily; for patients weighing 40 kg and greater, the dose is 40 mg by mouth daily.
- Vorasidenib should be swallowed whole with water at the same time each day without regard to food.
- If a dose is missed within 6 hours of usual time, patients should take the missed dose as soon as possible. If more than 6 hours have elapsed, the missed dose should be skipped, and patients should resume the regular schedule the following day.
- If vomiting occurs after taking a dose, patients should skip the day and resume the next dose the following day.
- For patients experiencing adverse effects from vorasidenib requiring dose adjustment, reductions are made in increments of 10 mg. If a patient is unable to tolerate 10 mg daily, then vorasidenib should be discontinued.
- The vorasidenib prescribing information includes the following dose adjustments based on hepatotoxicity:
- Grade 1 AST or ALT elevation: Does not require any dose adjustments; however, monitoring weekly hepatic labs is recommended until resolved.
- Grade 2 AST or ALT elevation: Following the first occurrence, vorasidenib should be held and then may be resumed at the same dose once the toxicity is ≤ grade 1 if recovery occurs within 28 days. If recovery occurs after 28 days, vorasidenib may be resumed at a reduced dose. For a recurrence, vorasidenib may be resumed at a reduced dose following resolution of hepatotoxicity to ≤ grade 1.
- Grade 3 AST or ALT elevation: Following the first occurrence, vorasidenib should be held and then may be resumed at a reduced dose if ≤ 28 days for the toxicity to resolve to ≤ grade 1. If recovery does not occur in ≤ 28 days or if Grade 3 hepatotoxicity recurs, vorasidenib should be discontinued.
- Grade 4 AST or ALT elevation: permanently discontinue vorasidenib.
- Grade 2 or 3 AST or ALT elevation with concurrent bilirubin elevation: Following the first occurrence, vorasidenib should be held and then may be resumed at a reduced dose once toxicity resolves to ≤ grade 1. For recurrence vorasidenib should be discontinued.
- For other adverse effects, the package insert recommends the following:
- Grade 3: Following the first occurrence, vorasidenib should be held until toxicity resolves ≤ grade 1, then may be resumed at a reduced dose. If the toxicity recurs, vorasidenib should be discontinued.
- If a patient experiences any grade 4 toxicities or adverse effects, vorasidenib should be discontinued.
- Drug interactions
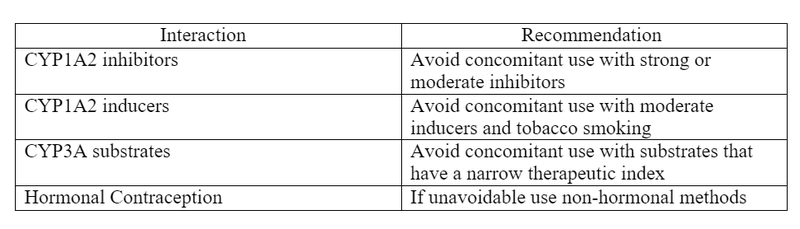
- Vorasidenib is an inducer of several CYP enzymes including CYP3A, and UGT1A4.
- Vorasidenib is primarily metabolized by CYP1A2.
- CYP1A2 -> inhibitors may increase vorasidenib plasma concentrations and inducers may decrease plasma concentrations.
- CYP3A -> vorasidenib use with CYP3A substrates may decrease the CYP3A substrate plasma concentrations
- Hormonal contraception -> may decrease hormonal contraception concentrations
Table 1. Drug Interaction Recommendations
- Pharmacists can assist in educating patients and/or caregivers on the risk of embryo-fetal toxicity and the preventative measures recommended to females of reproductive age and males.
- It is recommended for female patients of childbearing potential to use effective non-hormonal contraception during therapy and for 3 months after discontinuing therapy.
- It is recommended for male patients with female partners of childbearing potential to use contraception during treatment and for 3 months after discontinuing therapy.
Clinical Pearls
- Storage and Preparations
- Vorasidenib should be stored in a dry environment and at room temperature.
- Supplied in two strengths.
- 10 mg round tablet and 40 mg oblong tablet
- Liver function tests should be monitored prior to therapy, every 2 weeks during the first 2 months of therapy, then monthly for the first 2 years of therapy.1
- ServierONE patient assistance program for vorasidenib: Allows eligible patients to choose a program to help with financial assistance and more. Available at Vora_Patient_Enrollment_Form
References
1.VORANIGO (vorasidenib). FDA Package Insert. label (fda.gov) Accessed September 4, 2024.
2.Mellinghoff IK, Bent MJ, Blumenthal DT, et al. Vorasidenib in IDH1- or IDH2-mutant low-grade glioma (INDIGO). NEJM. 2023;389(7): 589-601.
3.Ruda R, Horbinski C, Bent M, et al. IDH inhibition in gliomas: from preclinical models to trials. Nat Rev Neurol. 2024;20: 395-407.
4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines(R)) for Central Nervous System Cancers V.3.2024. National Comprehensive Cancer Network, Inc. 2024. Accessed September 15, 2024.
5.Konteatis Z, Artin E, Nicolay B, et al. Vorasidenib (AG-881): A First-in-Class, Brain-Penetrant DualInhibitor of Mutant IDH1 and 2 for Treatment of Glioma. ACS Medicinal Chem Lett. 2020;11(2): 101-107.




