What is the potential role of Zenocutuzumab-zbco in the treatment of NSCLC or Pancreatic Adenocarcinoma?
- Zenocutuzumab-zbco is the first and only FDA approved systemic treatment for adults with NRG1 gene fusion in NSCLC or pancreatic adenocarcinoma. Zenocutuzumab-zbco was granted accelerated approval December 4, 2024 for 1) advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) harboring a neuregulin 1 (NRG1) gene fusion with disease progression on or after prior systemic therapy, or 2) advanced, unresectable, or metastatic pancreatic adenocarcinoma harboring an NRG1 gene fusion with disease progression on or after prior systemic therapy.1
- Zenocutuzumab-zbco is a bispecific IgG1 antibody targeting the extracellular domains of HER2 and HER3 expressed on cell surfaces, inhibiting HER2:HER3 dimerization and preventing NRG1 binding to HER3, leading to decreased cell proliferation and signaling through the phosphoinositide 3-kinase (PI3K)-AKT mammalian target of rapamycin (mTOR) pathway. Additionally, zenocutuzumab-zbco mediates antibody-dependent cellular cytotoxicity (ADCC).2
- The NCCN guidelines have been updated for Pancreatic Adenocarcinoma and Non-Small Cell Lung Cancer to include zenocutuzumab-zbco as a subsequent therapy option in patients with NRG1 fusion mutations.3,4
- Approval of zenocutuzumab-zbco was based on the eNRGy phase I/II open-label, multicenter, multicohort, trial (NCT02912949).2
- Patients included were ≥18 years old with locally advanced, unresectable, or metastatic solid tumor malignancy with a documented NRG1 fusion, had ≥1 measurable lesion, were previously treated with or unable to receive standard therapy and had recovered from toxicities of prior therapy, and had an ECOG PS ≤2. Laboratory screening values for eligibility include absolute neutrophil count ≥1.5 × 10⁹/l, platelets ≥100 × 10⁹/l, hemoglobin ≥8 g/dl, ALT and AST ≤3 × ULN (or ≤5 × ULN in cases of metastatic liver involvement), total bilirubin ≤1.5 × ULN (or ≤2 × ULN in cases of metastatic liver involvement) with exception of patients with Gilbert’s syndrome, and eGFR >30 ml/min. Select exclusion criteria include recent cancer treatment (≤14 days), NYHA class III or IV congestive heart failure, LVEF <50%, or a history of significant cardiac disease.2
- Eligible patients received zenocutuzumab-zbco 750 mg IV every 2 weeks
- At the time of accelerated approval, the major efficacy outcomes showed:
- In NSCLC: overall response rate (ORR) of 33% (95% CI: 22%, 46%) and median duration of response (DOR) of 7.4 months (95% CI: 4.0, 16.6)
- In pancreatic adenocarcinoma: ORR of 40% (95% CI: 29%, 59%) and DOR of 7.4 months (95% CI: 3.7, 16.6).
- Since the accelerated approval, updated results have been published evaluating the use of zenocutuzumab-zbco in 204 patients with NRG1 fusion-positive cancer (12 tumor types).
- The primary endpoint of the study was ORR.
- For patients with NSCLC (n=93), the ORR was 31% (95% CI: 22%, 39%)
- For patients with pancreatic adenocarcinoma (n=36), ORR was 44% (28%, 62%)
- The secondary endpoints included DOR, time to response (TTR), frequency and nature of adverse events (AEs), pharmacokinetics (PK) profile, and immunogenicity.
- DOR for NSCLC was 12.7 months (95% CI: 1.8, 29.5) and for pancreatic adenocarcinoma was 7.4 months (95% CI: 3.7, 16.6)
- Overall TTR: 1.8 months
- The most common adverse effects of any grade that occurred in >15% of patients were diarrhea, fatigue, nausea, anemia, and dyspnea. No grade 3 or 4 adverse events were identified in >5% of patients, though a low incidence of GI, skin, and cardiac toxic effects were seen and are known side effects of wild-type HER2 inhibition.
- The pharmacokinetic profile of zenocutuzumab-zbco was consistent with the profile reported for other humanized monoclonal antibodies. No relevant differences in PK profile were observed across tumor types.5
- Evaluations of overall survival (OS) and progression free survival (PFS) are ongoing with an estimated primary completion at the end of 2026.
- The primary endpoint of the study was ORR.
- Zenocutuzumab-zbco IV infusion is currently the only treatment indicated specifically for patients with NRG1 fusion genes in pancreatic cancer and NSCLC. Alternate agents that target HER signaling have also shown antitumor activity in NRG1 fusion–positive cancer. In a retrospective series involving patients with NRG1 fusion–positive NSCLC, 25% of those treated with afatinib had partial responses, with a median progression free survival of 2.8 months, and 60% had a best response of disease progression.6 A phase 2 study investigating treatment with seribantumab, an anti-HER3 IgG2 monoclonal antibody, showed a response in 8 of 22 patients (36%) with NRG1 fusion–positive cancer.7 There are currently no studies comparing zenocutuzumab-zbco with other agents, and PFS/OS results are currently ongoing.
What role can the pharmacist play in the management of patients on Zenocutuzumab-zbco?
- Pharmacists play an important role in managing care for patients on zenocutuzumab-zbco by managing appropriate initiation and access, dosing and preparation, and adverse event prevention, management and monitoring.
- Dosing and administration
- For both NSCLC and pancreatic adenocarcinoma, the dosing is 750 mg as an IV infusion every 2 weeks until disease progression or unacceptable toxicity. Zenocutuzumab-zbco is to be infused via peripheral or central line over 4 hours.
- Infusion-related reaction (IRR) prevention and monitoring
- Patients are to be monitored for at least one hour following completion of the first infusion. During subsequent sessions, infusion time may be reduced to 2 hours if there are no IRRs ≥ grade 2 during the first infusion.2
- Administer appropriate premedications (corticosteroids for first infusion, antipyretic, and H1 antihistamine). Corticosteroid premedication is optional after the initial infusion and may be utilized only for management.8
- Dose adjustments
- Dose adjustments for specific adverse reactions are detailed in table 1.
- There are no dose adjustments for zenocutuzumab-zbco based on renal or hepatic function, though patients with severely compromised renal or kidney function (ALT and AST ≤3 × ULN (or ≤5 × ULN in cases of metastatic liver involvement), eGFR >30 ml/min) were excluded from studies.2
Table 1. Adverse reactions of zenocutuzumab-zbco and associated dose adjustments8
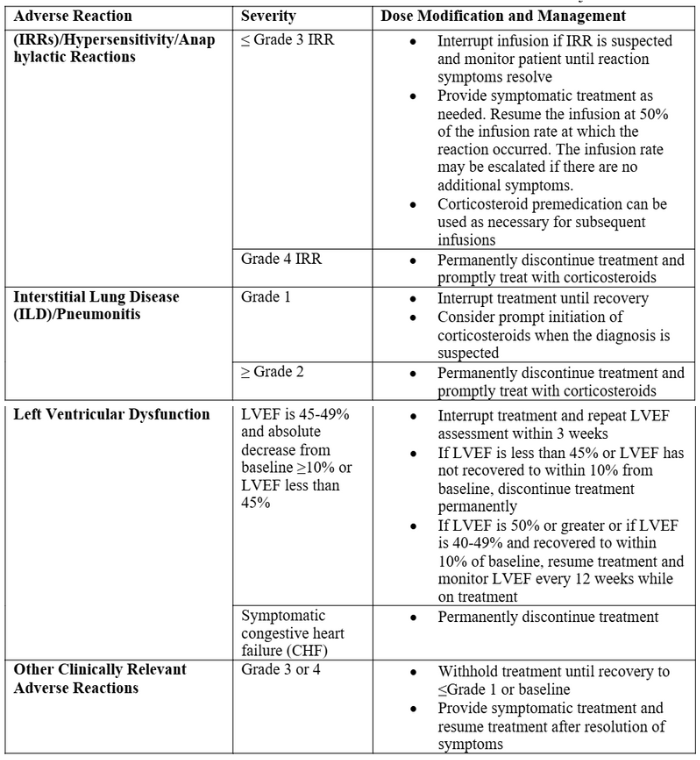
- Adverse reactions in patients with NSCLC
- The most prevalent AE (≥10%): diarrhea (25%), musculoskeletal pain (23%), dyspnea (18%), fatigue (17%), cough (15%), rash (14%), IRRs (12%), decreased appetite (11%), nausea (10%).
- Grade 3 or 4 AE (≥2%): dyspnea (5%), fatigue (2%), and diarrhea (2%).
- Grade 3 or 4 laboratory abnormalities (≥1%): increased gamma-glutamyl transpeptidase (5%), decreased magnesium (4.3%), decreased hemoglobin (4.2%), increased alanine aminotransferase (3.1%), increased aspartate aminotransferase (3.1%), decreased potassium (2.1%), decreased phosphate (1.1%).
- Adverse reactions in patients with pancreatic cancer
- The most prevalent AE (≥10%): diarrhea (36%), musculoskeletal pain (28%), nausea (23%), vomiting (23%), fatigue (21%), abdominal pain (18%), constipation (15%), IRRs (15%), edema (13%), hemorrhage (13%), abdominal distention (13%), stomatitis (10%), pyrexia (10%), anxiety (10%), and dry skin (10%).
- Grade 3 or 4 AEs (≥1%): diarrhea (5%), hemorrhage (5%), nausea (5%), abdominal pain (5%), fatigue (5%), vomiting (2.6%), musculoskeletal pain (2.6%).
- Grade 3 or 4 laboratory abnormalities (≥1%): increased gamma-glutamyl transpeptidase (15%), decreased sodium (10%), decreased platelets (10%), decreased hemoglobin (10%), increased alkaline phosphatase (8%), increased alanine aminotransferase (5%), increased aspartate aminotransferase (5%), increased bilirubin (5%), decreased phosphate (2.9%), decreased potassium (2.6%), decreased magnesium (2.6%), decreased leukocytes (2.6%).8
- Warnings and precautions due to treatment are given for the following:
- IRR: can cause serious and life-threatening IRRs, hypersensitivity and anaphylactic reactions. Zenocutuzumab-zbco should be administered in a setting with emergency resuscitation equipment and staff to monitor for IRRs. In clinical trials 13% of patients in trials experienced IRR with a time to onset of 63 minutes from the start of the 4-hour infusion.2,8
- ILD/pneumonitis: occurred in 1.1% of patients. Grade 2 ILD pneumonitis occurred in 0.6% of patients.
- Left Ventricular Dysfunction: Zenocutuzumab-zbco can cause left ventricular dysfunction, as is established in anti-HER2 therapies. Grade 2 LVEF occurred in 2% of evaluable patients and cardiac failure occurred in 1.7% of patients with 1 fatal event. Treatment has not been studied in patients with a history of clinically significant cardiac disease or LVEF less than 50% prior to initiation of treatment.
- Monitoring
- LVEF must be documented prior to initiation and monitored at regular intervals during treatment.
- In females of reproductive potential, baseline pregnancy status should be checked, and effective contraception should be utilized during treatment and for 2 months after last dose.
- Other monitoring throughout therapy: IRR, new or worsening pulmonary symptoms indicative of ILD/pneumonitis, CBCs at regular intervals, and response to treatment.8
- Drug interactions
- Concurrent use of zenocutuzumaz-zbco and any live vaccine is contraindicated as it may increase the risk of infection.10
Clinical Pearls
- NRG1 rearrangements are often not detected by DNA-based sequencing techniques because the large introns in NRG1 are not typically included in targeted next-generation sequencing panels or whole-exome sequencing. RNA-based sequencing is a superior method for identifying these alterations with low incidences of concurrent driver alterations.2
- NRG1 fusions are rare across different types of cancer, with a reported average incidence of <1% (between 0.27 and 0.5%).10 Two of the more prevalent incidence rates are in lung and pancreatic cancers: NRG1 fusions have been reported in 0.3–1.7% of lung cancer, 0.5–1.8% of pancreatic cancer.2
- Supply, storage and handling
- Each single-dose vial contains 375 mg/18.75 mL, and two vials are equivalent to one dose.
- Vials are to be stored in a refrigerator (2°C-8°C or 36°F-46°F) in the original carton to protect from light. Do not freeze or shake.
- Dilute and prepare zenocutuzumab-zbco as close to administration time as possible. If not used immediately, store the diluted solution refrigerated (2°C-8°C or 36°F-46°F) and protect from light unless the infusion is initiated within 2 hours of preparation.
- Diluted solution must be administered within 6 hours from end of preparation of infusion solution stored at room temperature (15°C-25°C or 59°F-77°F), or 28 hours from end of preparation of infusion solution stored refrigerated (2°C-8°C or 36°F-46°F).8
- Patient Assistance Programs: Drug has no formal patient assistance until it is officially on the market in 2025, at which point a formal patient assistance program will be accessible. To initiate a request, the patient's treating physician should contact Merus via email at EAP@merus.nl.
References
1.U.S. Food and Drug Administration. "FDA Grants Accelerated Approval to Zenocutuzumab-zbco for Non-Small Cell Lung Cancer and Pancreatic Cancer."
2.Kim DW, Schram AM, Hollebecque A, et al. The phase I/II eNRGy trial: Zenocutuzumab in patients with cancers harboring NRG1 gene fusions. Future Oncol. 2024;20(16):1057-1067. doi:10.2217/fon-2023-0824
3.National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 1.2025). Published February 2025.
4.National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 2.2025). Published February 2025.
5.Kim, D. W., Schram, A. M., Hollebecque, A., Nishino, K., Macarulla, T., Rha, S. Y., … Goto, K. (2024). Efficacy of Zenocutuzumab in NRG1 Fusion–Positive Cancer. N Engl J Med 2025;392:566-76. DOI: 10.1056/NEJMoa2405008
6.U.S. Food and Drug Administration. Ongoing Cancer Accelerated Approvals. Updated October 1, 2024.
7.Patil T, Carrizosa DR, Burkard ME, et al. CRESTONE: a phase 2 study of seribantumab in adult patients with neuregulin-1 (NRG1) fusion positive locally advanced or metastatic solid tumors. Cancer Res 2023; 83: Suppl 8: CT229. abstract.
8.Bizengri (zenocutuzumab-zbco) [package insert]. Merus N.V.; December 2024
9.Zenocutuzumab. In: Micromedex Solutions. IBM Watson Health; 2025.
10.Laskin J, Cabanillas ME, De Langen AJ, et al. NRG1 fusion-driven tumors: biology, detection and the therapeutic role of afatinib and other ErbB-targeting agents. Ann Oncol. 2020;31(12):1693-1703. doi:10.1016/j.annonc.2020.09.006



