What is the potential role for zolbetuximab-clzb in the treatment of locally advanced unresectable or metastatic G/GEJ adenocarcinoma?
- Zolbetuximab-clzb is a first-in-class chimeric IgG monoclonal antibody that binds Claudin 18.2 (CLDN18.2) and mediates cancer cell death via antibody-dependent cellular toxicity and complement-dependent cytotoxicity.1
- Claudin proteins are major structural components of tight junctions that regulate permeability, barrier function, and polarity of epithelial layers.
- CLDN18.2 is expressed in 35-40% of locally advanced unresectable or metastatic G/GEJ adenocarcinomas.2
- Zolbetuximab-clzb is FDA-approved in combination with fluoropyrimidine- and platinum-containing chemotherapy for the first-line treatment of adult patients with locally advanced unresectable or metastatic, HER2-negative gastric or gastroesophageal junction (G/GEJ) adenocarcinoma whose tumors are CLDN18.2 positive.1
- The efficacy and safety of zolbetuximab-clzb was evaluated in two parallel, multicenter, randomized, placebo-controlled trials: SPOTLIGHT and GLOW.3,4
- Inclusion: Patients aged ≥ 18 years with previously untreated CLDN18.2-positive, HER2-negative, locally advanced unresectable or metastatic G/GEJ adenocarcinoma with ECOG 0-1.
- Intervention:
- SPOTLIGHT: Zolbetuximab-clzb (800 mg/m2 loading dose followed by 600 mg/m2 IV q3w) + mFOLFOX6 (q2w). 1 cycle = 42 days. Oxaliplatin was dropped after 4 cycles.
- GLOW: Zolbetuximab-clzb (800 mg/m2 loading dose followed by 600 mg/m2 IV q3w) + CAPOX (q3w). 1 cycle = 21 days. Oxaliplatin was dropped after 8 cycles.
- Continued until disease progression, development of toxic effects, or start of another anticancer treatment.
- Results
- In both trials, zolbetuximab-clzb plus chemotherapy led to significantly longer median progression-free survival (mPFS) and median overall survival (mOS) compared to placebo plus chemotherapy.3,4
- Combined analysis10:
- mPFS 9.2 vs. 8.2 months (HR 0.71; 95% CI 0.61-0.83)
- mOS 16.4 vs 13.7 months (HR 0.77; 95% CI 0.67-0.89)
- The most common adverse events were nausea (76.0% vs. 56.2% and vomiting (66.8% vs. 34.2%)
What role can the pharmacist play in the management of patients on zolbetuximab-clzb?
- Pharmacists can play a role in the management of patients on zolbetuximab-clzb by ensuring appropriate dosing and administration and management of side effects.
- Dosing and administration of zolbetuximab-clzb:
- Initial doses should be started at a lower infusion rate, then increased as tolerated, as shown in Table 1.1
- Minimum infusion times for zolbetuximab:
- First infusion: 3.5 hours
- Subsequent infusions: 2.5 hours
- Minimum infusion times for zolbetuximab:
- Initial doses should be started at a lower infusion rate, then increased as tolerated, as shown in Table 1.1
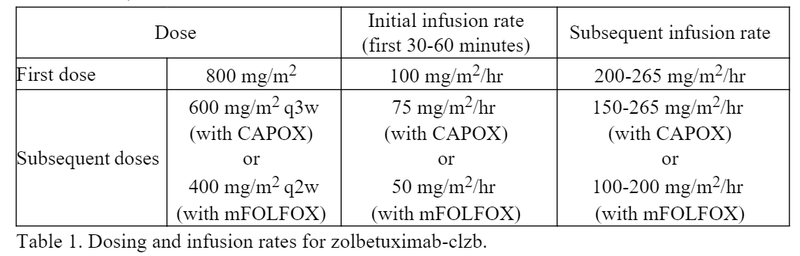
- Both the SPOTLIGHT and GLOW trials studied zolbetuximab-clzb given every 3 weeks. However, a clinical pharmacology study of zolbetuximab-clzb based on population pharmacokinetics found comparable safety and efficacy of two different dosing schedules: zolbetuximab-clzb 400 mg/m2 q2w to match up with mFOLFOX (given q2w), and zolbetuximab-clzb 600 mg/m2 q3w, as studied in SPOTLIGHT and GLOW, to match up with CAPOX (given q3w).6
- Management of nausea/vomiting:
- Severe nausea/vomiting has been seen with zolbetuximab-clzb. Nausea/vomiting is due to direct mucosal damage.10 It is worse with the first infusion and abates with subsequent doses.8 Nausea/vomiting is also correlated with faster infusion times.8 Pharmacists can help mitigate nausea/vomiting with antiemetics and infusion modifications.3,4,7
- Prevention of nausea/vomiting:
- Combination antiemetic prophylaxis should be administered 30 minutes prior to the infusion.1
- An expert panel on the management of nausea/vomiting for zolbetuximab-clzb recommends following the NCCN guidelines for high emetic risk.8
- Treatment of nausea/vomiting:
- Dopamine D2 receptor antagonists (i.e., prochlorperazine) were the most common rescue antiemetics in SPOTLIGHT and GLOW trials.3,4
- Pausing the zolbetuximab-clzb infusion for 30-60 minutes and/or slowing the infusion rate can also help alleviate nausea/vomiting.8
Clinical Pearls
- Dosage form: 100 mg single-dose vial for reconstitution.1
- Storage: Vials should be refrigerated at 2°C to 8°C (36°F to 46°F).1
- Once reconstituted, vials can be stored at room temperature 15°C to 30°C (59°F to 86°F) for up to 5 hours.
- The prepared infusion bag can be stored at room temperature for up to 6 hours or refrigerated for up to 16 hours.
- If the infusion time exceeds the recommended storage time, the infusion bag must be discarded and a new infusion bag prepared to continue the infusion.
- Immunohistochemistry (IHC) staining methods are used for the detection of CLDN18.2. In the SPOTLIGHT and GLOW trials, CLDN18.2-positivity was defined as ≥75% of tumor cells with moderate-to-strong (IHC 2+ or 3+) CLDN18 membranous staining.3,4
- The VENTANA CLDN18 (43-14A) RxDx Assay is FDA-approved as a companion diagnostic device to determine CLDN18.2-positivity.1
- Zolbetuximab-clzb has not yet been incorporated into the NCCN guidelines. Currently, the preferred first-line regimens for locally advanced unresectable or metastatic G/GEJ adenocarcinoma are:
- HER2 positive: Fluoropyrimidine (fluorouracil or capecitabine), platinum (oxaliplatin or cisplatin), and trastuzumab. Add pembrolizumab if PD-L1 combined positive score (CPS) ≥1.
- HER2 negative: Fluoropyrimidine and platinum. Add nivolumab if PD-L1 CPS ≥5 or pembrolizumab if PD-L1 CPS ≥1.
- MSI-H/dMMR: Pembrolizumab; dostarlimab-gxly; nivolumab and ipilimumab; or fluoropyrimidine, oxaliplatin, and nivolumab or pembrolizumab.
- Now, patients may have multiple first-line options that have not been studied head-to-head. The percentage of patients with advanced gastric/GEJ adenocarcinoma and co-expression of CLDN18.2 and other biomarkers is as follows:12-13
- CLDN18.2+ and HER2 positive: ~15%
- CLDN18.2+ and PD-L1 CPS ≥1: ~50%
- CLDN18.2+ and MSI-H/dMMR: ~8%
- The ongoing phase II trial, ILUSTRO will examine the efficacy and safety of zolbetuximab-clzb in combination with chemotherapy and PD-L1 inhibitors in patients with CLDN18.2-positive, HER2-negative locally advanced unresectable or metastatic G/GEJ adenocarcinoma.5
References
1.Vyloy (zolbetuximab-clzb) [prescribing information]. Northbrook, IL: Astellas Pharma US, Inc; October 2024.
2.Shitara K, Xu R-H, Moran DM, et al. Global prevalence of CLDN18.2 in patients with locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mg/GEJ) adenocarcinoma: Biomarker analysis of two ZOLBETUXIMAB phase 3 studies (spotlight and glow). Journal of Clinical Oncology. 2023;41(16_suppl):4035-4035.
3.Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133-2141.
4.Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial [published correction appears in Lancet]. 2023 Jul 22;402(10398):290.
5.Shitara K, Yamaguchi K, Shoji H, et al. Phase 2 trial of zolbetuximab in combination with mfolfox6 and nivolumab in patients with advanced or metastatic claudin 18.2-positive, HER2-negative gastric or gastroesophageal junction adenocarcinomas. Journal of Clinical Oncology. 2023;41(16_suppl).
6.Yang J, Yamada A, Klempner SJ, et al. Clinical Pharmacology profile of the claudin 18.2 antibody zolbetuximab. Journal of Clinical Oncology. 2024;42(3_suppl):316-316.
7.Shitara K, Pophale R, Matsangou M, et al. Management of nausea and vomiting (N/V) following first-line (1L) zolbetuximab + chemotherapy treatment in claudin-18.2 (CLDN18.2)+, HER2−, locally advanced (LA) unresectable or metastatic gastric or gastroesophageal junction (mg/GEJ) adenocarcinoma: Analysis from the phase 3 spotlight and glow studies. Journal of Clinical Oncology. 2024;42(3_suppl):372-372.
8.Samuel J. Klempner, Stephanie Braun, Sarah N. Gibbs, Rupali Fuldeore. Presented at the European Society for Medical Oncology Gastrointestinal Cancers (ESMO GI) Congress; June 26–29, 2024; Munich, Germany. Abstract 506PJasani B, Taniere P, Schildhaus HU, et al. Global Ring Study to Investigate the Comparability of Total Assay Performance of Commercial Claudin 18 Antibodies for Evaluation in Gastric Cancer. Lab Invest. 2024;104(1):100284.
9.Lordick F, Al-Batran SE, Ganguli A, Morlock R, Sahin U, Türeci Ö. Patient-reported outcomes from the phase II FAST trial of zolbetuximab plus EOX compared to EOX alone as first-line treatment of patients with metastatic CLDN18.2+ gastroesophageal adenocarcinoma. Gastric Cancer. 2021;24(3):721-730.
10.Shitara K, Shah MA, Lordick F, et al. Zolbetuximab in Gastric or Gastroesophageal Junction Adenocarcinoma. N Engl J Med 2024 Sep 26;391(12):1159-1162.
11.Kinugasa F, Kajikawa S, Weng J, et al. Effect of antiemetics on zolbetuximab-induced gastric injury and emesis in ferrets. J Pharmacol Sci 2024;156(3):161-170.
12.Kubota Y, Kawazoe A, Mishima S, et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open. 2023;8(1):100762.
13.Pellino A, Brignola S, Riello E, et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J Pers Med. 2021;11(11):1095



