FDA approves repotrectinib for ROS1-positive non-small cell lung cancer ǀ FDA
What is the potential role for repotrectinib in the treatment of non-small cell lung cancer (NSCLC)?
- Repotrectinib is an inhibitor of proto-oncogene tyrosine-protein kinase ROS1 and tropomyosin receptor tyrosine kinases (TRKs): TRKA, TRKB, and TRKC.1
- Repotrectinib is approved for the treatment of ROS1-positive locally advanced or metastatic NSCLC for patients who have previously received ROS1 tyrosine kinase inhibitors (TKIs) or are TKI-naïve. Approval was based on data collected in the ongoing single arm, global multicenter, open-label phase 2 TRIDENT-1 trial.1
- Primary efficacy outcomes were overall response rate (ORR) and duration of response (DOR).
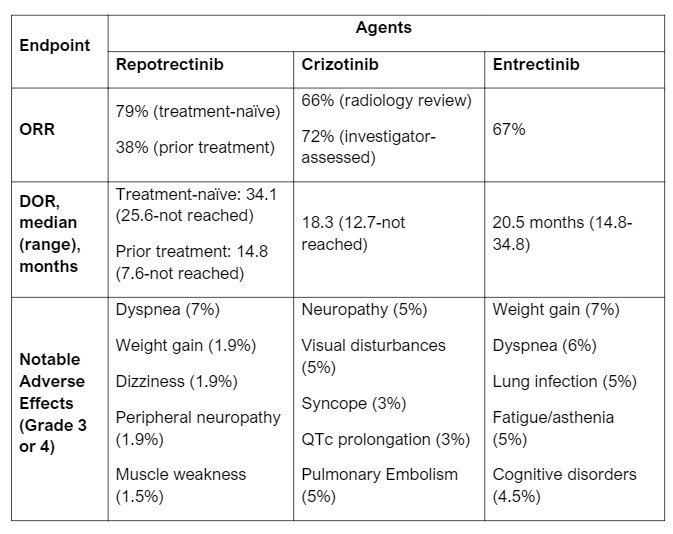
- Of the 71 patients in the ROS1 TKI-naïve group, confirmed ORR (cORR) was 79% (68-88% CI) while the 56 patients that received prior ROS1 TKI treatment (82% had crizotinib and 16% had entrectinib) had a cORR of 38% (25-52% CI). Median DOR was 34.1 months (25.6%-not evaluable CI) and 14.8 months (95% CI: 7.6, not evaluable) in the two groups, respectively.3
- 25.4% of patients in the ROS1 TKI-naïve group and 42.9% of patients with prior ROS1 TKI treatment had CNS metastases.
- Of the 8 treatment-naïve patients with brain metastases, 7 showed responses. In the prior ROS1 TKI group, 5 out of 12 patients showed responses.
Safety
- Common adverse effects include dizziness, dysgeusia, peripheral neuropathy, constipation, dyspnea, ataxia, fatigue, cognitive disorders and muscular weakness (details in Table 1).
- Central Nervous System (CNS) Adverse Reactions - In TRIDENT-1, CNS adverse reactions including dizziness, ataxia, and cognitive disorders occurred in 75%, 4% of which were Grade 3 or 4 events.
- Interstitial Lung Disease (ILD)/Pneumonitis - 2.9% of patients experienced ILD/pneumonitis (pneumonitis [2.6%]; ILD [0.3%]). Grade 3 ILD/pneumonitis occurred in 1.1% of patients.
- Hepatotoxicity - Increased alanine transaminase (ALT) occurred in 35%, increased aspartate aminotransferase (AST) occurred in 40%, including Grade 3 or 4 increased ALT in 2% and increased AST in 2.6%.
- Myalgia with Creatine Phosphokinase Elevation - Myalgia occurred in 13% of patients, with Grade 3 in 0.6%
- Hyperuricemia - 5% of patients experienced hyperuricemia with 0.9% experiencing Grade 3 or 4 hyperuricemia.
- Skeletal Fractures - Fractures occurred in 2.3% of patients. Fractures involved the ribs (0.6%), feet (0.6%), spine (0.3%), acetabulum (0.3%), sternum (0.3%), and ankles (0.3%).
Role in therapy
- As of this writing, the National Comprehensive Cancer Network (NCCN) recommends the following TKIs for the treatment of ROS1-positive advanced or metastatic NSCLC: ceritinib, crizotinib, entrectinib, lorlatinib and repotrectinib.2
- If a ROS1 rearrangement is detected, then first-line TKI treatment with entrectinib, crizotinib or repotrectinib is preferred over ceritinib. Lorlatinib remains as a second-line agent for disease progression. 2
- Repotrectinib and entrectinib share similar mechanisms of action as tropomyosin tyrosine kinase inhibitors.
- Repotrectinib has a potential place in therapy for ROS1-positive NSCLC alongside entrectinib and crizotinib. At the time of writing, there are no studies directly comparing repotrectinib with the other preferred treatment options, crizotinib and entrectinib. Patient populations in both efficacy studies for entrectinib and crizotinib did not delineate between treatmen-naïve or prior exposure to ROS1 TKI. ORR are similar between studies. See Table 1 below for more information on each agent.
- Currently, repotrectinib is recommended as one of the preferred agents, amongst entrectinib and crizotinib, for both advanced and metastatic ROS1-positive NSCLC.2 Repotrectinib possess a 90-fold greater potency than crizotinib for ROS1, which may lend to its use for refractory ROS1-positive NSCLC.7
What role can the pharmacist play in the management of patients on repotrectinib? 1
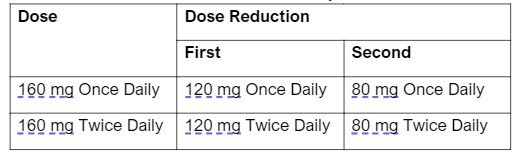
- Recommended dosage is 160 mg once daily for 14 days, followed by 160 mg twice daily, with or without food. It should be taken at approximately the same time every day.
- Monitoring drug-drug and food-drug interactions is important in ensuring safe drug concentrations are maintained and potential adverse effects are minimized.
- Strong and Moderate CYP3A Inhibitors – concomitant use may increase repotrectinib exposure and may increase incidence or severity of adverse effects.
- P-gp Inhibitors – concomitant use may increase repotrectinib exposure and may increase incidence or severity of adverse effects.
- Strong and Moderate CYP3A Inducers - concomitant use may decrease repotrectinib exposure, which may reduce efficacy of repotrectinib.
- CYP3A4 Substrates – repotrectinib is a CYP3A4 inducer which may decrease the efficacy of CYP3A4 substrates, including itself. Hormonal contraceptives can be ineffective while taking repotrectinib; it is recommended to utilize non-hormonal contraceptives during treatment.
- There are specific dose modifications for CNS toxicity, hepatotoxicity, CPK elevation, ILD/pneumonitis and intolerable grade 2 or grade 3+ AE.
Table 2: Recommended Dose Reductions for Repotrectinib Adverse Reactions
Clinical Pearls
- Repotrectinib is currently available as a 40 mg capsule.
- Repotrectinib must be stored at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C to 30°C (59°F to 86°F).
- Advise patients to inform their healthcare provider if they notice any new or worsening CNS symptoms, pulmonary symptoms, muscle pain, signs or symptoms associated with hyperuricemia, and hepatotoxicity.
- For financial or co-payment assistance program for repotrectinib, patient can enroll in assistance program through Bristol-Myers Squibb to be assisted with out-of-pocket costs, patient assistance program enrollment, or bridging program enrollment, available at Augtyro BMS Access Support.
References
1.Augtyro (Repotrectinib) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company 2023.
2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 1, 2024. Accessed January 8, 2024. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
3.Lin JJ, Drilon A, Byoung Chul Cho, et al. Intracranial and systemic efficacy of repotrectinib in advanced ROS1 fusion-positive (ROS1+) non-small cell lung cancer (NSCLC) and central nervous system metastases (CNS mets) in the phase 1/2 TRIDENT-1. Journal of Clinical Oncology. 2023;41(16_suppl):9017-9017. doi:https://doi.org/10.1200/jco.2023.41.16_suppl.9017.
4.Rozyltrek (Entrectinib) [package insert]. San Francisco, CA: Genentech, Inc. 2023.
5.BMS Access Support® | AUGTYROTM (repotrectinib). www.bmsaccesssupport.bmscustomerconnect.com. Accessed December 13, 2023. https://www.bmsaccesssupport.bmscustomerconnect.com/augtyro#.
6.Xalkori (Crizotinib) [package insert]. New York, NY: Pfizer Inc. 2023.
7.Byoung Chul Cho, Drilon A, Doebele RC, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). Journal of Clinical Oncology. 2019;37(15_suppl):9011-9011. doi:https://doi.org/10.1200/jco.2019.37.15_suppl.9011
8.Drilon A, Chiu C-H, Fan Y, et al. Long-term efficacy and safety of entrectinib in ROS1 fusion-positive NSCLC. JTO Clin Res Rep 2022;3(6): 100332.