
HOPA and PTCE will host Oncology Pharmacists Connect on June 20-21 at Downright Austin, A Renaissance Hotel, in Austin, Texas. There will be 10+ live credit hours presented plus 4 HOPA-Curated BCOP CEs.
Get a full description of Oncology Pharmacists Connect.
HOPA Presents Curated BCOP CEs
HOPA has curated a half-day of in-person education that features some of the latest science and most sought-after BCOP learning available so far this year.
Register Here
Tentative Schedule: HOPA-Curated BCOP CEs
- All courses are on Friday, June 21, 2024 and listed in Central Time
- Please see below for full course descriptions and learning objectives
- Please note: Program content subject to change based on new data presented at ASCO Annual Meeting
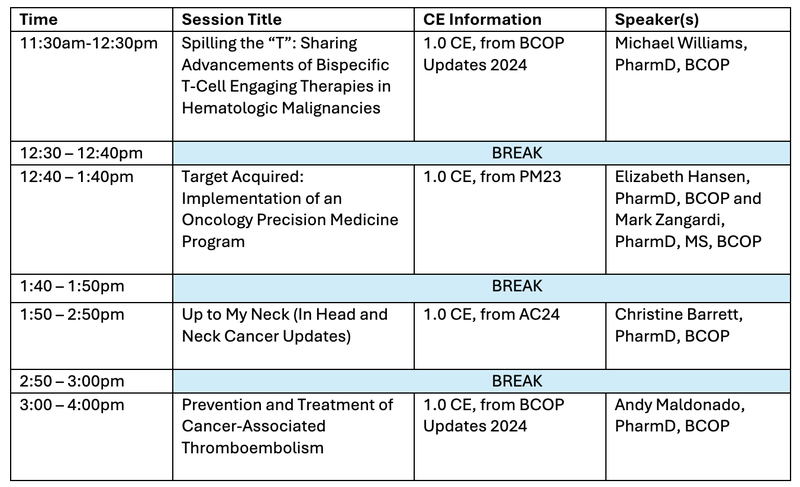
- All 4 BCOP/ACPE credits must be claimed in HOPA Learn by no later than Monday, August 5, 2024.
Credits & Cost
- Credit Type: BCOP
- Start Date: June 21, 2024
- Last Purchase Date: June 21, 2024
- Member Price: $99
- Non-Member Price: $149
- BCOP Credit Amount: 4
- Credit Claim Expiration Date: August 05, 2024
Register Here!
Course Descriptions and Learning Objectives
- UAN# 0465-0000-24-083-L01-P
- Activity for Pharmacists
Author: Michael Williams, PharmD, BCOP
Session Description: This session aims to address updates in the development of bispecific antibody (BsAb) therapies for hematologic malignancies. Granted accelerated approval in 2014, blinatumomab was the first BsAb therapy option for acute lymphoblastic lymphoma (ALL). In recent years additional therapies have received approval for diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), and multiple myeloma (MM) with even more investigational agents on the horizon for these indications as well as others such as acute myeloid leukemia (AML).
Given the mechanism of action, these agents often pose safety concerns similar in nature to chimeric antigen T-cell (CAR) therapy, but also may introduce unique side effects. The session contents will provide an overview of each FDA-approved BsAb including study population and safety concerns. Supportive care, administration logistics, and cost will also be addressed.
Learning Objectives:
- Identify the mechanistic target and indication for each FDA-approved BsAb therapy among hematologic malignancies
- Recall package insert recommendations to aid decision-making for the appropriate administration setting.
- Provide supportive care recommendations for the prevention and management of BsAb-related adverse events in multiple myeloma
- Apply knowledge of CRS and ICANS to compare risks between BsAbs and CAR T-cell therapy
- UAN#: 0465-0000-24-077-L04-P
- Activity for Pharmacists
Authors: Mark Zangardi, PharmD, MS, BCOP and Elizabeth Hansen, PharmD, BCOP
Session Description: Cancer treatment is increasingly defined by biomarkers and genomic alterations, commonly known as precision medicine . Application of precision medicine is variable across institutions, with some employing formal programs, and others without dedicated infrastructure. Participants will evaluate their organization's approach to oncology precision medicine. The presenters will review the importance of precision medicine in oncology and strategies for implementation of a dedicated program, including justification for pharmacist involvement.
Learning Objectives:
- Describe the importance of biomarker and genomic testing in routine cancer care
- Define essential components, steps for creation, and best practices of a health system-based oncology precision medicine program
- Analyze various tools and training programs to support the practice of precision medicine
- Justify the inclusion of pharmacists in developing, launching, and maintaining a successful oncology precision medicine program
- UAN#: 0465-0000-24-078-L01-P
- Knowledge Course for Pharmacists
Author: Christine Barrett, PharmD, BCOP
Session Description: Head and neck cancer is the seventh most common cancer worldwide, accounting for 1.5% of all cancer deaths in the United States. In patients with advanced disease, the overall prognosis is poor, and there is a need to better optimize treatment. This presentation will focus on data in the recurrent and metastatic setting, as well as ongoing studies in both early and advanced stage disease. Recently published data will be interpreted, including studies on chemo-immunotherapy in nasopharyngeal carcinoma. This presentation will provide guidance on how to tailor first-line therapy in patients with advanced disease. Lastly, we will discuss ongoing trials that are being conducted, which include the evaluation of immunotherapy in the perioperative setting, and novel therapy combinations.
Learning Objectives:
- Review current guidelines for the treatment of recurrent and metastatic head and neck cancers
- Interpret the latest data in recurrent and metastatic head and neck cancer
- Recommend appropriate treatment for recurrent and metastatic head and neck cancer based on patient- and disease-specific factors
- Discuss ongoing clinical trials for systemic therapy in head and neck cancer
- UAN#: 0465-0000-24-081-L01-P
- Activity for Pharmacists
Author: Andy Maldonado, PharmD, BCOP
Session Description: Cancer-associated venous thromboembolism (CAT) is a major cause of morbidity and mortality in patients with cancer. There have been several recent studies comparing direct oral anticoagulants (DOACs) with low-molecular weight heparin (LMWH) in the management of CAT. This session will discuss recent updates to guidelines for the prevention and management of CAT and will review data comparing the efficacy and safety of DOACs versus LMWH in the treatment of CAT.
Learning Objectives:
- Identify patients with cancer who may benefit from primary thromboprophylaxis
- Compare data for use of direct oral anticoagulants (DOACs) versus low-molecular weight heparin (LMWH) in the treatment of cancer-associated thrombosis (CAT)
- Select the most appropriate anticoagulant for treatment of CAT given patient-specific characteristics
- Discuss duration of treatment for CAT
Important Information about these Courses
Technology requirements: HOPA Learn requires a modern web browser (Internet Explorer 7+, Mozilla Firefox, Apple Safari, Google Chrome) and the ability to listen to audio with the content.

HOPA is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education.
In order to receive ACPE credit, the pre-test, post-test, and course evaluation must be completed.. In order to claim BCOP credit, you must pass the BCOP post-test with a 75% or higher.
All CE hours will be transmitted to the CPE Monitor and BPS within 1-2 weeks of course completion.